Nathan R. York and Dr. Gregory F. Burton, Microbiology
The search to understand Human Immunodeficiency Virus (HIV) and the progression of the disease that it causes, AIDS, has led scientists to delve further and further into the intricacies of viral mechanics. Although not considered living organisms by many scientists, these entities manage to find ways to enter, replicate, and thrive within the cells of their hosts. As we study any one particular virus, insights into many viruses can be gained.
HIV attacks the immune system of its host and, as a result of its replication processes, depletes and eventually destroys human immune function. This disease progression is a very interesting one. Not long after the infection is established, the host goes through a latent period of disease wherein very little virus can be detected in the blood. However, there are large amounts of viruses being produced in secondary lymphoid tissues, such as the spleen and lymph nodes. The highest concentrations are found on the surfaces of follicular dendritic cells (FDCs).
FDCs serve a specific function in the immune system. Their purpose is to trap and present antigens to B cells in order to elicit an antibody response. In HIV infection, however, they may serve another purpose. Although they themselves are not infected by HIV, they contribute in some fashion to an upregulation of viral protein production. It has recently been shown that when CD4+ T cells are cultured in the presence of FDCs there is an increase in the amount of viral protein, specifically p24, a protein found in the viral capsid, or shell. There are several different hypotheses by which this may occur. The purpose of this study was to explore one of them; namely, the upregulation of transcription of viral genes.
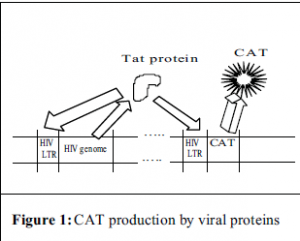
In order to achieve this purpose, a two-part experiment has been designed. In the first part, a retroviral transduction system was created by using genes encoding viral structural proteins to make a viral shell. This shell is then used to transport a reporter gene into the desired cell. For this experiment, the DNA carrying the reporter gene also carried the HIV LTR. This is the region of DNA to which cellular proteins attach and initiate the processes leading to production of the virus’ proteins i.e. p24. With this type of construct, one can measure the activity of the promoter (the HIV-LTR) through the amount of protein encoded by the reporter gene that is present. In this case, that protein is an enzyme called chloramphenicol acetyl transferase, or CAT, which is used in many systems like this one and can be measured using a radioactivity assay.
Once the retrovirus was created, it would then be used to infect primary blood lymphocytes. After this infection, the cells would then be infected with HIV and cocultured with and without FDCs. The HIV would provide the cell with a viral protein called Tat, whose function is to bind to the LTR and enhance transcription. The Tat would not only bind to the LTR that came from the HIV, but also the LTR that came from the retroviral construct and CAT production would begin (see Fig. 1). If the FDCs do in some way upregulate the transcription of the viral proteins, then we should see an increase in the amount of CAT in those cells cocultured with FDCs over those that were not.
Initially, we acquired three separate plasmids (small, circular pieces of DNA) from Dr. Barbara Felber at the National Cancer Institute. Two of these plasmids carried a specific gene necessary to make the viral shell. The other plasmid carried the LTR-CAT construct that the virus would deliver to the cells. These plasmids had been used before to make a retroviral construct1. We tried for several months to insert all three of these plasmids into cultured cells. Various known techniques were used, but with no success. Inserting plasmids into cells using any method is not very efficient. Getting just one plasmid only has a 30 – 50% success rate. Getting three plasmids in at the same time proved too difficult in our experience.
In our next attempt to establish this system, we used a commercially available ΦNX (Phoenix) cell line. This is a packaging cell line that already contains the viral structural protein genes. Hence, the only plasmid that had to be inserted was the reporter construct. This greatly improved our chances of success. As with any new procedure, the details of optimal efficiency had to be worked out. Using a different reporter assay, it was found that we could insert the plasmid into the cells with moderate success (data not shown).
An attempt was then made to infect primary T cells with the virus-containing supernatant from the ΦNX cells. After the cells were infected with the retroviral construct, half of them were then infected with HIV and allowed to incubate for various amounts of time ranging from 0-60 hours. The cells that were not infected with HIV showed a slight increase in CAT concentration over a 48-hour period, proving that the construct had successfully entered into the cell and was being randomly transcribed. The cells that were infected with HIV, however, showed no CAT increase (data not shown). A possible reason for this could be that the amount of HIV used to infect the cells was too high and it overwhelmed and destroyed them.
In order to complete this experiment, the optimal HIV concentration must be determined and the experiment should be reproducibly redone in order for any data to be acceptable. The data may then be used to help us understand, at a molecular level, the intimate relationship between HIV and follicular dendritic cells.
References
- Felber BK, Pavlakis GN. A quantitative bioassay for HIV-1 based on transactivation. Science. 1988 Jan 8; 239 (4836): 184-7.