Richard Turley and Drs. Richard A. Robison and Gregory F. Burton, Microbiology
Due to recent findings that HIV replication can persist even with highly active anti-retroviral therapy (HAART), it has become increasingly necessary to develop new therapies that can be used as adjuncts with HAART, to combat HIV infection. One possible alternative strategy is to block infection by down-regulation of the cell receptors needed for viral entry. Amara, et al. proposed that HIV coreceptor down-regulation by stromal cell derived factor 1-a (SDF-1a), the physiological ligand of CXCR4, blocks infection of target cells by X4 strains of HIV-1 (1). Mack, et al. demonstrated that Aminooxypentane-RANTES (a derivative of RANTES, one of CCR5’s physiological ligands) induces CCR5 internalization and inhibits normal receptor recycling. They proposed that down-regulation of CCR5 by Aminooxypentane-RANTES may be an effective strategy for inhibiting entry of R5 strains of HIV-1 (2). Phorbol esters (activators of Protein Kinase C) such as Phorbol-12-Myristate-13-Acetate (PMA) and Phorbol Dibutyrate (PDBu) have been shown to down-regulate the surface expression of HIV-1 receptor CD4 and coreceptor CXCR4, and thus inhibit HIV-1 infection (3,4,5,6,7). These compounds have proved useful tools in elucidating the mechanisms of CXCR4 and CD4 regulation; however, due to their tumor-promoting activities, the use of phorbol esters to down-regulate HIV receptors and thus inhibit viral entry has been largely ignored. Because Prostratin is a non-tumor-promoting phorbol ester, we studied the anti-HIV effects of this compound (8,9,10,11).
My study had the following objectives: 1. Determine if Prostratin down-regulates CD4 and CXCR4 in neoplastic and primary T cells. 2. Determine if this down-regulation is mediated by PKC. 3. Determine if this down-regulation blocks HIV-1 entry into target cells.
To accomplish this, I used the following experimental design. CEM-SS (a lymphoblastic CD4+ T cell line) and two other T cell lines (PM1 and HUT 78) were treated with Prostratin for 24 hours. The CD4 and CXCR4 receptor levels on these cells were then analyzed with flow cytometry. CD4+ peripheral blood lymphocytes (CD4 + PBLs) were isolated from healthy donors, and these cells were treated and analyzed as above. Because these cells are the in vivo target cells of HIV-1 it was important to determine if Prostratin down-regulated CD4 and CXCR4 in these cells. Finally, CEM-SS cells were pretreated with GF-109203X, a PKC-specific inhibitor, treated with Prostratin, and then analyzed by flow cytometry to determine the role of PKC in Prostratin-induced down-regulation of these receptors.
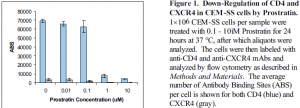
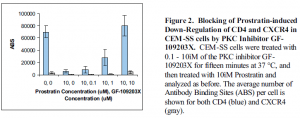
Prostratin treatment induced down-regulation of both CD4 and CXCR4 surface expression in CEM-SS cells (Figure 1). Similar results were attained with HUT 78 and PM1 cells (data not shown). Maximal down-regulation occurred within four hours, and complete re-expression occurred within twelve hours after removal of Prostratin from culture (data not shown). CD4 and CXCR4 were also down-regulated by Prostratin treatment in CD4+ PBLs in a manner similar to that observed in CEM-SS cells (data not shown). Importantly, HIV-1IIIB entry was blocked by Prostratin treatment in a dose-dependent manner (Figure 2). Prostratin-induced down-regulation of CD4 and CXCR4 was abrogated by pretreatment with GF-109203X, a Protein Kinase Cspecific inhibitor (Figure 3). From these results, the following conclusions can be made: 1. Prostratin induces the rapid down-regulation of CD4 and CXCR4 in both CEM-SS neoplastic T cells and CD4+ PBLs. 2. Prostratin-induced down-regulation of these cellular receptors inhibits HIV-1 entry into target cells. 3. Prostratin-induced down-regulation of CD4 and CXCR4 appears to be mediated by Protein Kinase C. 4. Down-regulation of HIV-1 receptor and coreceptors may be a potential therapeutic strategy that merits further research.
Figures
Works Cited:
- Amara, A., et al. J. Exp. Med. 186, 139 (1997).
- Mack, M., et al. J. Exp. Med. 187, 1215 (1998).
- Parolini, I., et al. J. Biol. Chem. 20, 14176 (1999).
- Ruegg, C. L., et al. J. Biol. Chem. 26, 18837 (1992).
- Signoret, N., et al. J. Cell Sci. 111, 2819 (1998).
- Signoret, N., et al. J. Cell Sci. 3, 651 (1997).
- Firestein, G. S., et al. Cellular Immunol. 113, 63 (1988).
- Gustafson, K. R., et al. J. Medic. Chem. 35, 1978 (1992).
- Gulakowski, R. J., et al. Antiviral Res. 33, 87 (1997).
- Szallasi, Z., et al. Canc. Res. 51, 5355 (1991).
- Szallasi, Z., et al. Canc. Res. 53, 2507 (1993).