Stanton Nielsen and Dr. Brad Berges, Department of Microbiology and Molecular Biology
Many scientists are skeptical of using humanized mice to study the human adaptive immune response. The reasoning behind this skepticism stems from discrepancies in various studies seen previously. A study conducted by Traggiai et al. showed that humanized mice elicited a human adaptive immune response when challenged with the tetanus toxin (Traggiai). In addition, good antibody responses have been shown in humanized mice when challenged with Dengue virus (Kuruvilla). However, others have found only rare antibody responses to HIV when humanized mice were challenged (Baenziger). As mentioned earlier, a study conducted by Becker et al. showed the production of human monoclonal IgM antibodies, but they were not able to detect any class switching to human IgG (Becker). Seeing the variability in previous studies, the hope of my project was to better the consistency within the immune response of humanized mice. I planned to achieve this by monitoring concentrations of human antibodies in humanized mice over various time points. I also worked with another undergraduate in determining the process of B cell development and maturation so that we could better predict which humanized mice will respond to an antigen and produce the best antibody response. Through improving the humanized mouse model, our goal was to help researchers obtain more reliable and reproducible results when conducting research with humanized mice.
In conducting my research I collected over 80 samples through bleeding mice for serum samples. However, in collecting all of these samples, I ran into a number of hurdles. Multiple times I began bleeding the mice at three weeks following engraftment to collect samples and would bleed them three times over a six-week period of time before I would screen the mice for percent peripheral engraftment. The reasoning behind this is I wanted to discover whether or not there were indeed detectable levels of antibody production during this time. However, it is standard protocol to screen mice for percent peripheral engraftment at eight weeks. Screening before this time period could lead to a false negative concerning mouse engraftment of human immune cells. This being the case, the majority of my samples had to be discarded because almost all of the mice screened had no engraftment. Over the last year, we have only had one litter of mice that have had a percent peripheral engraftment of over 25 percent. This being the case, the goals of my research have not yet been accomplished.
Thus far, I have collected positive samples from one litter of mice, but I have yet to run ELISA tests on these samples because it was only last week that we received confirmation on their engraftment. I plan to test these samples as well as continue to bleed these mice to check for serum antibody levels over the coming months.
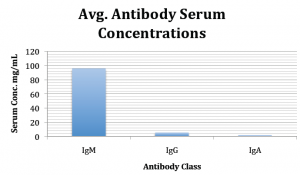
Although I have not been able to test any of the mice that I engrafted for the purpose of this study, I did screen mice that were engrafted earlier for a previous study. These mice were 16 weeks following engraftment so approximately the middle time point of my study. The following graph illustrates the serum antibody levels according to each antibody class for the ten mice screened.
From this data I have been able to present along with my collaborator German Cuadra at the American Society of Microbiology Intermountain branch meeting and I have also had my research accepted for presentation at the upcoming Autumn Immunology Conference in Chicago, Illinois.
As mentioned earlier, I ran into many problems when screening mice that were not engrafted. This being an enormous problem I have decided to begin screening mice at the same time points as previously proposed, but instead not restricting myself to only using the same mice throughout the study for every time point. This will be helpful in my study because I will be able to make progress on many of the later time points while I continue to work on screening the mice at the initial time points. This will also help me to further my project and publish at an earlier date.
Although I have not yet reached my previous goals, I have made an admirable effort and will continue to work on this project until publication. With new insight into this research and a more efficient way to carry out this study, I will further the knowledge of the immune system in humanized mice and will use this data to determine the optimal time to test the immune response in humanized mice.
References
- Baenziger S, Tussiwand R, Schlaepfer E, Mazzucchelli L, Heikenwalder M, Kurrer MO, Behnke S, Frey J, Oxenius A, Joller H, et al: Disseminated and sustained HIV infection in CD34+ cord blood cell transplanted Rag2-/-{gamma}c-/- mice. Proc Natl Acad Sci USA 2006, 103:15951-15956.
- Becker PD, Legrand N, van Geelen CM, Noerder M, Huntington ND, Lim A, Yasuda E, Diehl SA, Scheeren FA, Ott M, et al: Generation of human antigen-specific monoclonal IgM antibodies using vaccinated “human immune system” mice. PLoS One 2010, 5:e13137.
- Berges, B.K. and M.R. Rowan, The utility of the new generation of humanized mice to study HIV-1 infection: transmission, prevention, pathogenesis, and treatment. Retrovirology, 2011. 8: p. 65.
- Kuruvilla JG, Troyer RM, Devi S, Akkina R: Dengue virus infection and immune response in humanized Rag2-/-gc-/- (RAG-hu) mice. Virol 2007, 369:143-152.
- Traggiai, E., et al., Development of a human adaptive immune system in cord blood cell-transplanted mice. Science, 2004. 304: p. 104-107.