Joshua Nicholson and Dr. Brad Geary, Department of Plant and Wildlife Sciences
Review
This project seeks to establish and understand a link between virulence and phytotoxin production between varying strains of Pyrenophora semeniperda, a necrotrophic fungal pathogen that attacks cheatgrass (Bromus tectorum). Cheatgrass is an intermountain west invasive weed that is outsourcing native plants and damaging ecosystems from desert to montane. Cheatgrass monocultures can increase fire frequency which can occur as often as 3-5 years. P. semeniperda is a naturally occurring fungal pathogen that can cripple a cheatgrass seed before germination with the use of a secondary metabolite such as a phytotoxin. Predation of this type is effective in destroying the seed bank of cheatgrass and limiting its yearly production. Our team is focused on understanding how this fungal pathogenesis works and if it is possible to form a mycoherbicide that can be used to eradicate cheatgrass monocultures.
Previous research has shown that strains of P. semeniperda vary in virulence, the ability to produce and effectively utilize its phytotoxin. Also, studies has shown that there is a negative correlation between virulence and growth rate. One hypothesis for this correlation is that producing these phytotoxins is costly and that there is a trade off between resource and phytotoxin production. The proposal outlined in the ORCA was searching for an answer to this hypothesis; are phytotoxin production and virulence positively correlated?
Data
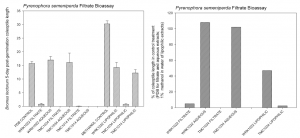
To test this hypothesis, we outlined a process to perform secondary metaboliteextractions on varying strains. Fungal strains were grown in liquid culture by potato dextrose broth and solid culture on wheat seeds. The liquid, or filtrate, cultures have been extracted and their phytotoxins were weighed and analyzed. The extractions on filtrate cultures have shown that the production of phytotoxin is different among each strain. We are still in the process of comparing this information with that of virulence. For example, because the strain produces more phytotoxins, does this mean it will yield a higher toxicity in a seed test? A corollary seedling bioassay study has been performed on several of these metabolite extractions (see chart) to assess the phytotoxicity on dormant cheatgrass seeds. We test raw filtrate (liquid culture of P. semeniperda), lipophilic extraction, and other experimental variables on dormant seeds and we measure the growth of each seed by the coleoptile.
Conclusion
Bioassay studies have shown that phytotoxicity activity is in the lipophilic fractions (fatloving) from the filtrate extractions. A secondary metabolite extraction on fungal filtrate strains separates the secondary metabolites from the two phases of aqueous and lipophilic. The graph shows that the lipophilic fractions are the meanest at reducing coleoptile growth and germination. Aqueous fractions were least effective and compare closely to our control. This confirms that there is phytotoxicity within the lipophilic fractions of the filtrate extractions. We are in the process of collaborating with Dr. Evidente, an organic chemist, on isolating and purifying the unknown secondary metabolites in our filtrate strains. Dr. Evidente has extracted this pathogen before and has identified a phytotoxin called cytochalasin B in the solid cultures. The seedling bioassay study has shown us that there is activity in filtrate cultures and we are very curious to identify the unknown phytotoxin present.
Discussion
We have revealed clues to the secondary metabolite activity within strains of P. semeniperda but we are far from understanding our alternate hypothesis that virulence and phytotoxin production are positively correlated. We still need to analyze more strains from filtrate cultures and perform further bioassay studies. Solid culture metabolite extraction has not yet been performed and this will be our next big step. The reason for our absence on solid culture data was that our extraction protocol lead us into an emulsion. An emulsion is when the two phases mix and it is difficult to separate. Marco Masi, a Phd student with Dr. Evidente, came to BYU recently and explained a technique to reduce the risk of future solid culture emulsions. One simple way to remedy the issue is to add more solvent to the extraction. We did run into other shortcomings that have limited our progression into this research. Our pathology lab was not equipped for metabolite extractions and we had to set up our lab space and equipment from scratch with a Rotovapor, a nitrogen tank for solidifying extractions, and general lab equipment.
In summary, we have verified that a phytotoxin is present in the lipophilic fraction of the filtrate that has previously not been researched. Further metabolite extraction on filtrate and solid culture strains still needs to be performed alongside a bioassay study. Identification of the unknown metabolites in the lipophilic fraction needs to be analyzed. This work is being spearheaded by Dr. Evidente at the University of Naples Italy who is familiar with the pathogen P. semeniperda.
Acknowledgement
We would like to thank ORCA for their generous donation that has helped fund this research. When we come closer to understanding our alternate hypothesis, this study will be coauthored with Dr. Antonio Evidente and his Phd student Marco Masi. I will be pursuing a Masters Degree in Environmental Science and this research will support my thesis.
References
- Evidente, A. et al. “Cytochalasins Z1, Z2, and Z3, three 24-oxal[14]cytochalasans produced by Pyrenophora semeniperda.” Phytochemistry. 60. (2002) 45-53
- Susan, M.E., T.E. Stewart, S. Clement. “The quick and the deadly: growth vs virulence in a seed bank pathogen.” New Phytologist. 187. (2010): 209-216.