Jonathan Walter Dukes and Dr. Ronald Leavitt, Department of Microbiology
Bacterial high adherence is an important of research because of its effect on bacterial pathogenesis and intestinal colonization. In the laboratory of Dr. Ronald Leavitt, we have been studying the ability of certain bacteria to prevent the colonization of intestinal epithelial cells by other bacteria. Of particular interest to us are non-pathogenic Escherichia coli strains that have the ability to out compete and overrun colonies of Salmonella, as well as the colonies of the pathogenic E. coli strains that cause the turkey disease colibacillosis. Such E. coli have the potential to be used as an interfering agent against Salmonella and pathogenic E. coli strains found in turkeys. Currently, high amounts of antibiotics are used to limit and control Salmonella, which is not particularly pathogenic to turkeys, and the population of virulent E. coli strains. The use of antibiotics in the turkey feed could be greatly reduced through the use of an interfering agent for bacterial control.
The ability of certain E. coli to act as interfering agents is believed to occur through a combination of two traits. The first trait is the ability to produce the bacteriotoxin microcin. Microcin is a peptide of about 6000 daltons that exhibits either bacteriocidal or bacteriostatic effects upon a wide variety of Enterobacteriaceae (1). We believe that the production of this protein aids the E. coli by killing the surrounding bacterial strains that are susceptible to the microcin, thereby opening up epithelial cell for it to colonize.
The second trait that aids these E. coli strains in interference is the ability to tightly adhere to the epithelial cells of the intestine. This adherence is very important to intestinal colonizing bacteria because with out it, the bacterium stands little chance of staying attached to intestine as the digesting food is pushed through. As a result of this pressure, different strains of E. coli have developed different mechanisms to facilitate adherence. We have shown through assays that quantify bacterial adherence that different mechanisms exhibit varying degrees of success. We have also shown through bacterial counts of intestinal swabs that those that strains that adhere best to the epithelial cells more easily colonize the intestine. One of the best adhering strains of E. coli that we have found has been named strain 28. This strain has a plasmid that encodes the high adherence proteins. Great strides towards achieving this goal have been made through bacterial conjugation, but we do know the sequence of the high adherence genes encoded on the plasmid. Obtaining this knowledge will help us to identify other E. coli strains that are using the same genes for adherence, as well as broadening our understanding the mechanism of adherence. We also want the gene sequence so we can optimize the plasmid that the genes are residing on. Currently, we estimate that the plasmid to be 106 kilobase pairs (kbp) in size, which is 100 fold larger than what is needed. We believe that shrinking the plasmid size will help the bacteria to keep the plasmid because large plasmids are very expensive, energy wise, to replicate. My task was to shrink down the plasmid to an optimal size that could then be sequenced.
I hypothesized with this project that the plasmid could be cut down to size through a series of restriction digests, ligations and reinsertions. I began by isolating the plasmid from strain 28 by using a Quiagen plasmid preparation. This gave me sufficient quantities of plasmid DNA for my restriction digests. I chose two restriction enzymes for my cuts, Not I and Sfi I. These were chosen because they are both rare cutting enzymes. After many trials, Not 1 was deemed to be unsuitable as it was discovered to cut the plasmid in only one location. Sfi I was much more promising because it cut the plasmid into two segments. After cutting the plasmid with Sfi I, I relegated each segment onto itself using a T4 ligase. This formed two smaller plasmids. There was little difficultly in separating the two smaller plasmids because only one of the plasmids had an origin of replication. Only plasmids with origins of replicationwill be copied and passed to both daughter cells during cell division. It is our belief that the high adherence gene is located near the origin of replication so the plasmid that was kept and replicated by the cell should contain the high adherence gene.
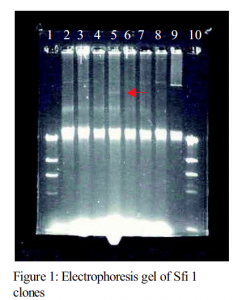
Once the new smaller plasmids were produced, they were then reinserted into the E. coli lab strain XMR blue. This was done by electroporation. Electroporation is a cloning technique that uses a strong electric current to make holes in the side on the bacteria so they will take up the plasmid DNA. Electroporation is not perfectly efficient so the cells must be tested for the presence of the plasmid. This was the major hurdle for the project. Normally, simple antibiotic tests are used to identify the plasmid carrying cells. Our high adherence plasmid does not appear to have any sort of genes for antibiotic resistance. This means that it was necessary to perform many plasmid mini preparations. Despite their ability to be done in ten minutes, the magnitude of the number of test that needed to be preformed made this a large task. After many trials, one cells with a smaller plasmid was identified (see figure 1). The band in lane 9 of figure 1 is the original large high adherence plasmid. Lane 5 contains the Sfi 1 clone containing a smaller plasmid (the band indicated by the red arrow). Unfortunately, it also appears to have a larger plasmid similar in size to the uncut plasmid in lane 9. High adherence assays of the clone in lane 5 shows that it has moderate adherence compared to the high adhering parent strain. This leads me to believe that the larger plasmid is not the original, or has lost the high adherence genes. It also means that the smaller plasmid my have lower gene expression resulting in lower adherence. The Sfi 1 fragment will need to be recloned in order to verify this.
This project did not take us as far as we had hoped, but it did teach us many things. It taught us that inserting a cut fragment of the high adherence plasmid into a non-adhering E. coli will pass on the adherence trait. Therefore not all of the plasmid is necessary for adherence and the gene is near the origin of replication. Further work must be done in order to get the plasmid down to a size that will permit sequencing the gene, and that work can proceed using this method.
References
- Leavitt, RW, C Braithwaith, MM Jensen. 1997. Colicin V38 and Microcin C38 Produced by Escherichia Coli Strain 38. Avian Diseases. 41:568-577.