Jesse Cobell and Dr. John Kauwe, Department of Biology
Importance of the Project
In 2011, the Alzheimer’s Association released in their annual facts and figures that 5.4 million people suffer from Alzheimer’s disease. Alzheimer’s is the sixth leading cause of death in the United States and is the only disease in the top ten that cannot be prevented or cured. The report explains that diagnosis is a significant difficulty and emphasizes the importance of identifying another method such as serum biomarkers to use in the diagnosis of the disease (Alzheimer’s Association). The ability to diagnose early or detect risk factors before onset would make an enormous impact on management of the disease for patients and family members. It would also allow doctors to provide improved care and researchers an opportunity to develop drugs or therapies that target the specific proteins discovered in these experiments.
Progress of the Project
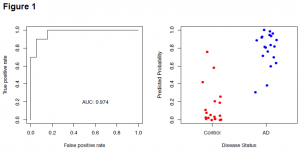
Thus far, the project has been very successful. Setbacks like the mass spectrometer being broken on and off for a period of months, have kept the project from being fully completed in the ideal timeline. A few months back we received more data from our statistician that showed incredibly significant results from the original set of data (Fig 1). These ROC curves show the statistical significance of a small group of the potential biomarkers. Anything about a .90 is considered extremely significant, but these curves show .974. This has made the validation project extremely exciting because if we get similar results this would be an incredible discovery. Currently, most of the new validation samples from Washington University have been run through the mass spectrometer and a large number have already been analyzed with the Analyst software. We will be looking for significant differences in peptide biomarkers between Alzheimer’s patients and controls. This time around the data is being analyzed completely blind to avoid any bias on the analysis side. Once the analysis is complete for all of the validation samples, biomarkers that do not appear in at least 85% of samples will be discarded for lack of consistency. We hope to see the same significant biomarkers show up in the validation in order to strengthen our results.
Academic Outcomes
If the validation results show biomarkers as significantly correlated between cases and controls as the original set of samples, then this paper could be published in a very high profile journal. A poster of the preliminary results has already been presented at the 2012 Alzheimer’s Association International Conference in Vancouver, Canada.
Conclusion
Despite problems with the original timeline, this project is going extremely well and we hope to see the final results come to fruition very soon. A key part of BYU’s mission is applying secular knowledge to solve real-world issues. Developing a serum blood test for Alzheimer’s will change the lives of individuals and families everywhere by enhancing understanding and opening the possibility of creating a cure for this devastating disease. Our results could help pave the way for these incredible possibilities.
Acknowledgements
I offer a special thanks to Dr. John Kauwe who has been an incredible mentor and inspiring scientist. I must also thank Dipti Shah, the graduate student on the project and our collaborator Dr. Steven Graves. I am also very grateful for the help of my fellow undergraduates, Frederick Rohlfing, Tyler Mower, and Jeff Olson.
References
- Alzheimer’s Association. 2011 Alzheimer’s Disease Facts and Figures. Alzheimer’s & Dimentia: The Journal of the Alzheimer’s Association. March 2011; 7:208-44.