Stephanie Carlson and Dr. Bradford Berges, Department of Microbiology and Molecular Biology
Science rarely cooperates as anticipated, but that can often lead to unexpected insight and solution. I ran into that this semester with a certain aspect of our project. We are continuing to collect data for the American Society for Virology in late July and a future publication. We will need more replicates for a publication, but we have optimized our approach and protocol.
Instead of using a GFP positive virus that should have been fairly simple to use, we had to infect cells with wild type virus. For some reason, the GFP positive virus could not be effectively isolated. We had chosen the GFP positive strain in the first place because it would be easy to see which cells were infected using only a fluorescence microscope. However, when faced with the challenge of unfruitful cell culture infection, we had to switch tactics. We ordered an antibody against one of the late viral proteins of the HHV‐6, gp116. We ordered a secondary antibody against the first with a FITC fluorochrome attached. This way, we can control for non‐specific binding in addition to being able to determine which cells harbored infectious particles.
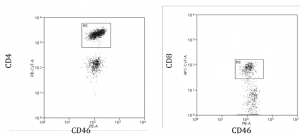
Recently, we have conducted several tests with infected cells and the aforementioned antibody setup. We collected promising data and hope to show which types of cells are getting infected and where they are located in the body. This semester included optimizing our qPCR protocol to measure viral load and investigating cell targets of HHV‐6. We looked specifically at human blood cells, humanized mouse blood cells, and PBMCs. The main targets of HHV‐6 are helper T cells, which are CD4 + and often found in the blood, spleen, bone marrow, and lymph nodes. Cytotoxic T cells are less of a target, but still important. The cellular receptor for HHV‐6 is thought to be CD46, which we found to be present on CD4 + T cells and CD8 + T cells (see fig. below). This data shows blood from a humanized mouse contains both CD4 + and CD 8+ T cells with the cellular marker CD46. We are still confident in our choice to use humanized mice as a model.
We will continue to investigate target cells and locations, and the ex vivo infection itself will occur this week. We are very excited about our new approach and hope it proves to be successful. We are confident that our findings will be consistent with our hypothesis and will provide us with publishable results.