Spencer Bell and Dr. Jeffrey Edwards, Department of Physiology and Developmental Biology
Carrying out my research project with my mentor’s assistance has provided valuable experiences and important lessons. At the present time, however, my research has not produced the results we had anticipated. We had hoped to demonstrate that a certain receptor in the brain called transient receptor potential vanilloid 1 (TRPV1) modulated memory formation in the developing brain by influencing a process known as long-term depression, or the weakening of a chemical connection between two brain cells. Although we have not yet produced statistically significant results, our observations indicate that we may simply need to conduct more experiments to acquire enough data and large enough sample sizes to reach statistical significance. Now that we have refined our techniques to more effectively acquire data, we hope to reach this point soon.
One of the major difficulties we encountered in our research was due to our use of neo-natal age rodents in our experiments. Our experiments involved the induction of long-term depression in rat brain slices via electrical stimulation. The cells in these slices can be used out of the body by keeping them in oxygen-enriched artificial cerebrospinal fluid (ACSF). Slices typically remain healthy in this solution for 7-8 hours. We found, however, that slices from neo-natal rats were more difficult to keep healthy in these conditions than those from mature rats. After some initial failures, I began to search the scientific literature to determine if other researchers who performed similar studies on immature brain slices used experimental methods different from ours. Although most papers described methods that were not significantly different from our own, I eventually found a paper that described using ACSF with a high sucrose content during the slicing of immature rat brains.1 My mentor explained that the sucrose can have a neuro-protective function and is sometimes used to improve slice health during slicing. Once we started using this high-sucrose solution during slicing, it was much easier to obtain healthy slices that would produce good results.
Another factor that inhibited our research was the constraints placed on us by rat ages. Previous studies suggested that a developmental switch in the memory mechanisms in a rat’s brain occurs 14 days after birth. Because we wanted to focus on the role TRPV1 played in memory formation before this developmental switch, we were limited to using only rats that were 14 days old or younger. Breeding protocols and gestation periods did not always agree with our scheduling, and our laboratory technician would typically only use one animal per day for the various experiments our lab was performing. Consequently, young rats were not always available when we wanted, and occasionally other research projects would take priority when they were available. This made our research somewhat slow-going. To remedy this, I asked our laboratory technician to train me in IACUC approved methods of brain slicing. Once I could perform the slicing myself, it was easier for me to have the slices I needed when rats of the appropriate agegroup
were available.
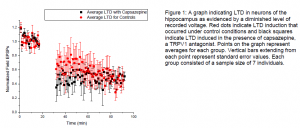
To determine the role of TRPV1 in the area of the brain we were interested in, the hippocampus, we applied a chemical called capsazepine to the brain slices as we electrically induced LTD in them. Capsazepine is a TRPV1 antagonist, meaning that it blocks the receptor’s natural activation when applied. If TRPV1 indeed modulated LTD in the hippocampal region we
studied, we expected to see differences in the magnitude of the average LTD observed in slices that received capsazepine during LTD induction and those kept under control conditions. The figure below shows the data we have collected thus far. This figure appears to indicate negative results. Although a general trend of difference is observed, it is not yet a difference that would be considered statistically significant.
We anticipate that with continued data collection, the sample sizes in the control and capsazepine groups will be large enough to more clearly indicate a difference between them if it exists. We are also experimenting with rats that are younger than those we used previously; we have considered the possibility that the developmental switch that has been observed in rat development may come at a time that varies between individuals and may be earlier than we anticipated.
In summary, although we encountered multiple setbacks in our research and are still unable to show conclusive evidence supporting our hypothesis, we are hoping to acquire data in the next month or two that will allow us to draw more firm conclusions. We hope that our additional experimentation will prove our hypothesis correct, but if not, even negative results will have contributed to the understanding of the cellular mechanisms underlying memory formation. If we do obtain sufficient evidence to uphold our hypothesis, we will determine whether any follow-up experimentation is necessary and begin the process leading to publication. If our results are definitively negative, we will perhaps include them as a sort of addendum to another relevant publication from a different research project.
References
- Nosyreva, E.D. and Huber K.M. (2005). Developmental Switch in Synaptic Mechanisms of Hippocampal Metabotropic Glutamate Receptor-Dependent Long-Term Depression. Journal of Neuroscience 25, 2992-3001.