Steven Bray and Professor David Busath, Department of Zoology
1,3-di(1-pyrenyl)propane(hereinafter dipyrenylpropane) has proven very useful as a fluorescence probe in determining fluidity and monitoring phase transitions in biological membrane systems.1 By virtue of its structure, dipyrenylpropane may fluoresce light in an excimer or a monomer configuration. Each configuration fluoresces light at a different wavelength, thus allowing us to monitor the excimer-to-monomer intensity ratio. The excimer form occurs when, as the light is absorbed, the pyrene branches come within close proximity, transferring energy and fluorescing light back at a different wavelength.2 The monomer configuration is defined by the lack of energy transfer and a consistent fluorescence wavelength. Therefore, as membrane fluidity is increased it allows the pyrene branches to come in closer contact and increases the excimer-to-monomer ratio. Consequently, a low excimer-to-monomer ratio would correspond with low fluidity. Modeling dipyrenylpropane and simulating its behavior could prove valuable in future studies of membrane fluidity.3
The goal of my project included modeling dipyrenylpropane and determining at which conformations the excimer fluorescence occurs. By calculating the lowest energy conformations of dipyrenylpropane a map could be plotted to designate distinct low energy wells. As the molecule moves from one low energy well to the next the subsequent conformations would be probable candidates for the excimer configurations. Thus, the first step was to determine the lowest energy conformations.
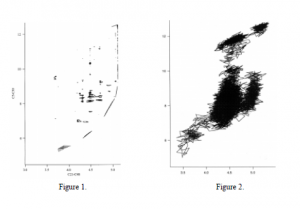
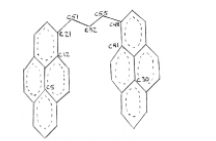
Using Quanta, I produced a model of the molecule and minimized the energy. I continued by writing a program in CHARMM (Chemistry at HARvard Macromolecular Mechanics) that systematically rotated four primary dihedral angles and the fourteen corresponding dihedral angles. Each of the four primary dihedral angles was progressively rotated 30 degrees forming 20,736 possible conformations. At each conformation the energy of the molecule was calculated as well as the distance from C5-C30 and C21-C48. These distances showed the relative proximity between the near ends of the pyrenes and the middle of the pyrene rings. Initially it was assumed that when both distances, C5-C30 and C21-C48, were small and the pyrenes were close together they would indicate the excimer configuration. As with any programming, initial plans are often delayed with unexpected bugs and miscalculations. I used Quanta to verify the accurateness of the final results of my program. Of the 20,736 conformations I selected the 1,000 with the lowest 36 energies to form a contour plot as seen in figure 1. The x-axis and y-axis represent the distances between C21-C48 and C5-C30, respectively. The contour represents the conformations with energies from 55-60.
After mapping the conformations I used Quanta to perform a molecular dynamics run with dipyrenylpropane. The langevan dynamics was run for 100,000 steps at 900 Kelvin with each step corresponding to .001 picosecond. The simulation recorded the frequency that the molecule was in a specific conformation. In figure 2., the most frequently occupied conformations are shown in black. The highly occupied conformations should closely correspond to the conformations with the lowest energy, which it does when compared to figure 1.
Also with the aid of Quanta I plotted the most frequent positions of the four primary dihedral angles within the simulation timeframe. The results of this analysis showed that the angles C12- C21-C51-C52 and C52-C55-C48-C41 remained constant and the angles C21-C51-C52-C55 and C51-C52-C55-C48 had approximately three regular positions. These results need to be more firmly solidified by making more dynamics runs.
At this point in our research we discovered that based on laboratory experiments the excimer state of dipyrenylpropane may remain in that configuration for as long as 100 nanoseconds.2 Because the dynamics simulations operate with such small time intervals it would be impossible to monitor the change between excimer and monomer without occupying a computer for an incredible amount of time.4 The length of time in the excimer state makes it virtually impossible to run significant dynamics simulations that would be of any substantial value. Therefore, our initial plan of action reached an impasse until we can devise a method to work around this obstacle.
References
- Zachariasse, Klaas. Intramolecular Excimer Fluorescence as a Probe of Fluidity Changes and Phase Transitions in Phosphatidylcholine Bilayers. Chemical Physics Letters. 1980. 73:6-11.
- Dr. John Bell, Brigham Young University, Zoology Dept.
- Dr. David Busath, Brigham Young University, Zoology Dept.
- Dean Anderson, Undergraduate advisor