Jeremy K. Beard and Dr. Eric Jellen, Agronomy and Horticulture
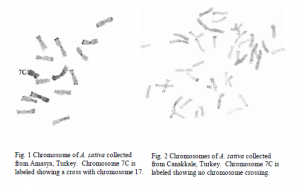
Previous studies have shown that chromosomal rearrangements largely influence changes in the genetic information found in the genus Avena (oat). Examinations of approximately 90 accessions of A. sterilis, the wild ancestor of A. Sativa and A. byzantina, using C-banding and molecular genetic markers previously revealed Turkey and Upper Mesopotamia to be the likely center of origin of the two cultivated oat taxa (2). E. N. Jellen identified a chromosome crossing between chromosomes 7C and 17 which predated the domestication of cultivated hexaploid (six copies of each chromosome) oat taxa A. sativa and A. byzantina( 1).
My research consisted of fifty cultivars and landraces of A. sativa and A. byzantina, specifically looking at chromosomes 7C and 17 to trace the geographic distribution of this translocation within and around the center of origin. My hypothesis was that the region responsible for 7C-17 chromosome crossing restricted recombination within adjacent blocks of genes which together confer adaptive advantage to A. sativa under a spring growth habit. Thus, landrace oats growing under spring cultivation should have the 7C-17 chromosome cross (Fig. 1 and Fig. 2). The results of this experiment confirmed my hypothesis.
The process of C-banding, a technique used to stain chromosomes, was used to study the chromosome rearrangements in the two oat taxa. To do this, the seed was germinated on filter paper adding hydroxyurea and treflan in a series of steps to control the cell cycle of the root cells. The root cells were then squashed onto a specimen slide and stored in a freezer. Staining of the chromosomes was achieved by immersing the slides in absolute ethanol for 20 min. and then in .2 M HCl at 59 degrees Celsius for 90 s., rinsed in distilled water, and immersed in saturated barium hydroxide at room temperature for 7 min. After rinsing the slides to remove the barium, the slides were incubated in 2X SSC (.3 M trisodium citrate) at 59 degrees Celsius for 40 min. and then placed in a staining solution (Giemsa/ 6.8 pH buffer) at room temperature until appropriately stained and then rinsed in distilled water. Slides were allowed to air dry before affixing cover slips using Permount. Point pictures of the chromosomes were taken at this point using a CCD digital camera attached to a Zeiss photo microscope. The digital camera is attached to a PC with Zeiss image analysis software, enabling accurate analysis of the chromosomes.
References
- Jellen, E. N., Gill, B. S., Fox, S. L., Wilson, W. A., and McMullen M. S. Translocations in current and ancestral spring and winter oat accessions. Agronomy Abstracts p.78. American Society of Agronomy, Madison, WI. (Proceedings of ASA 1996 Annual Meetings. Indianapolis, Indiana 1997. Relationships Nov. 3-8, 1996.)
- Zhou, X., Jellen, E. N., and Murphy, J. P. Between cultivated oat and Avena sterilis based on molecular and cytogenetic techniques. Crop Science (in preparation).