Tyler Prestwich and Dr. Michael Stark, Physiology and Developmental Biology
To better understand embryonic events leading to the determination of limb position along the anterior-posterior (A-P) axis, we compared pre-limb bud stage developmental morphology of three vertebrate organisms: chicken, goose and swan. These organisms were selected because of the differences each exhibit with regard to the A-P location of the forelimbs, or more specifically, the number of cervical vertebrae. By observing disparities between the embryonic morphologies of the different species, we were able to identify specific morphological distinctions that correlate with A-P patterning. Based on the data obtained, we hypothesize a mechanism whereby a delay in embryonic “differentiation” in relationship to somite segmentation may result in A-P patterning differences. Therefore, we believe that the relative timing of key embryonic events is critical in determining A-P limb position.
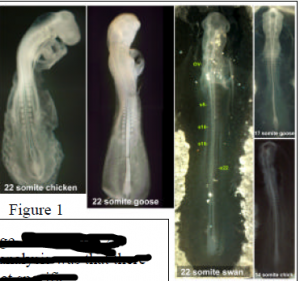
The data we obtained supports a model wherein the timing of at least some developmental events are likely to be critical to the general morphology and A-P patterning of the animal, including placement of the limbs. Figure 1 shows chick, goose and swan embryos each with 22 pairs of somites. Note the difference in overall morphological development. The right side of figure 1 also highlights the differences in somite number of embryos at the same developmental stage. Note that when staging embryos by morphological characteristics (not by somite number), a stage 11 chick has 14 somites, while a stage 11 swan has 22 somites. An overall observation stemming from this analysis was that there were significant differences in the number of somites at specific morphological stages of development when comparing organisms with a different number of cervical segments (i.e. a swan with the three primary brain vesicles clearly visible exhibited 20+ pairs of somites, while a chicken with similar head morphology exhibited 10+ pairs of somites). This developmental delay in morphology compared to somite number corresponds very well with differences in the number of cervical vertebrae in the adult organisms.
In addition to head morphology, we also examined the development of the heart at different stages in the three organisms. These observations were in agreement with what was detected in the head. When looking at all three organisms with hearts at the same developmental stage, we observed that the number of somites varied between species (not shown). We also found that while there is no difference between the rate of heart movement from the head toward the future body cavity when comparing the chick and the swan, the initiation times of that caudal movement are significantly different between the two species. This suggests that heart development, including caudal movement of the heart, is delayed in organisms with a greater number of cervical somites. This developmental delay corresponds with the model presented below.
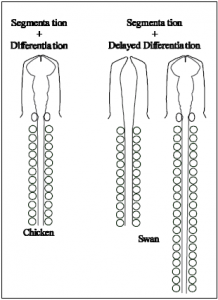
Here we present a model of A-P patterning based on the hypothesis that somite segmentation occurs independently from the timing of developmental differentiation. According to our model shown at the right, organisms with more cervical vertebrae (swan) experience a delay in differentiation while somite segmentation from the pre-somitic mesoderm occurs in the absence of significant developmental advancement. Organisms with fewer cervical vertebrae (chicken) experience less of a developmental delay. We hypothesize that organisms that initiate “differentiation” later (delayed differentiation) generate more somites prior to an undetermined patterning/differentiation event, and that this in turn results in a more caudal placement of secondary embryonic structures such as the limbs. Fine tuned patterning would follow (such as refinement of hox gene expression boundaries) finalizing the organism’s overall morphology.
Current and futur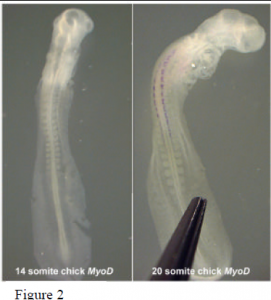 e studies hope to expand our understanding of developmental timing by looking more closely at the timing of gene expression, particularly markers of differentiation. Preliminary analysis of somite differentiation was performed using in situ hybridization where embryos of varying stages were probed for MyoD expression. Figure 2 shows two representatives (14 and 20 somite chick embryos). The 20 somite embryo exhibits MyoD expression down to the 14th somite. Somites in the younger embryo, however, have yet to differentiate as indicated by the absence of MyoD expression. This observation suggests a delay in differentiation at the molecular level while somite segmentation continues. Then, in response to some developmental cue, several of the rostral somites begin to differentiate almost simultaneously. Later expression of MyoD in the more caudal somites seems to occur in concert with somite segmentation. Future experimentation with molecular differentiation markers will shed more light on the mechanisms acting to establish the A-P axis of vertebrate embryos.
e studies hope to expand our understanding of developmental timing by looking more closely at the timing of gene expression, particularly markers of differentiation. Preliminary analysis of somite differentiation was performed using in situ hybridization where embryos of varying stages were probed for MyoD expression. Figure 2 shows two representatives (14 and 20 somite chick embryos). The 20 somite embryo exhibits MyoD expression down to the 14th somite. Somites in the younger embryo, however, have yet to differentiate as indicated by the absence of MyoD expression. This observation suggests a delay in differentiation at the molecular level while somite segmentation continues. Then, in response to some developmental cue, several of the rostral somites begin to differentiate almost simultaneously. Later expression of MyoD in the more caudal somites seems to occur in concert with somite segmentation. Future experimentation with molecular differentiation markers will shed more light on the mechanisms acting to establish the A-P axis of vertebrate embryos.
