Rachelle R. Olsen and Dr. Kim L. O’Neill, Microbiology and Molecular Biology
Angiogenesis, the development and differentiation of new blood vessels from pre-existing vasculature, is tightly controlled during physiological processes including wound healing, growth, and development. Angiogenesis is also associated with several human malignancies including arthritis, psoriasis, and cancer. Angiogenesis, which provides a source of nutrients and a waste outlet for biological products, is absolutely required for a tumor to grow larger than a few millimeters in size. Blood vessels formed by angiogenesis are young and immature and can facilitate metastasis, the spread and subsequent growth of primary tumor cells to distal body sites.
To study the mechanisms involved in angiogenesis and metastasis, I used a chick embryo model system. Due to its simplicity and low cost, this model is widely used to gather preliminary data. Animal models provide real tissue barriers to cell invasion and metastasis, which yield results with greater physiological significance. After a detailed project proposal was submitted to the IUCAC office for approval, I was able to begin my research.
To model angiogenesis, chick embryos were removed from their shells after two days of incubation and placed into sterile weigh boats. On day three of incubation, embryos were treated with 2.5% methylcellulose patches containing either VEGF (vascular endothelial growth factor) a potent angiogenesis inducer, or a negative control. On day six, the embryos were removed from the incubator and the entire CAM (chorioallantoic membrane) containing the developing vasculature was photographed with a digital camera.
Last year I used the CAM model to show that a plant extract from Convolvulus arvensis may have anti-angiogenic properties. One of the main problems I had with this project was that I had no way to quantify my results. To solve this problem, I collaborated with David Tomer, another student in our lab, to develop a computer program which would accurately quantify blood vessels within the CAM. I treated and photographed all of the embryos, and David worked on designing the computer program. After several months of refining the program, we believe that we now have the system to quantify our results. When analyzing an image, the program will go through the following procedure. First, the image of the embryo is subtracted from the digital photograph, insuring that differences in embryo size cannot skew the results. Next, a threshold limit is selected to identify blood vessels and to minimize background. The image is then skeletonized generating a black and white image. The program will then quantify the total length of blood vessels in the image as well as the number of branch points, two parameters commonly used to quantify angiogenesis. We are currently working on further ways to refine and improve our analysis program such as adding a way to estimate the total blood vessel area shown in the image. Hopefully we will soon be able to show a significant difference in the organization of blood vessels in VEGF-treated embryos as compared to the negative control.
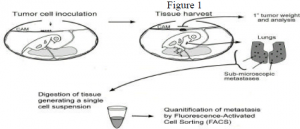
The second goal of my project was to develop a reliable chick embryo model system to study events in metastasis. Our metastasis protocol was based on that reported by Zijlstra, et.al. (Cancer Research, 62: 7083-7092, 2002).
To model metastasis, the CAM was dropped using pressure from a mild vacuum, causing the air sac to be displaced from the end of the egg to the top of the egg. A one-centimeter square window was drilled above the displaced air sac. On day six of incubation, twenty-five ìL of a single cell suspension containing 1×106 MCF-7 cells was inoculated onto the CAM. MCF-7’s, a human cancer cell line, were used since breast cancers tend to metastasize to the lungs. Embryos were then incubated for an additional eight days allowing a primary tumor to develop on top of the CAM and for spontaneous metastastes to develop in the lungs. On day fourteen, embryos were dissected and the primary tumor and the lung tissue were harvested. Lung tissue was treated with collagenase to generate a single cell suspension. This solution was then analyzed by fluorescence activated cell sorting (FACS) to quantify the number of human cells which had invaded the lung tissue. This model will allow us to compare the metastastic potential of cells after treatment with a suspected metastasis inhibitor. This procedure is summarized in Figure 1 below.
My time working on this project was spent developing and optimizing a protocol to treat the embryos and obtain the lung tissue. My original plan was to use MCF-7’s transfected with GSP. I then planned to verify my results using direct fluorescent microscopy to visualize GSP positive cells in the chick lung tissue. After I finished my part of the project, several students from our lab continued to work with this model system. Currently they are using a new method to quantify metastasis, in which levels of human thymidine kinase are measured in the lung suspension. They are also planning to verify their results using RT-PCR, or real time reverse transcriptase polymerase chain reaction. This technique is more sensitive and FACS analysis relies on detection of human mRNA in the lung tissue suspension. This method is also quantitative allowing precise measurements of the level of metastasis. Our lab hopes to continue using chick embryos as model systems for both angiogenesis and metastasis as we study inhibitors of these two pathological processes.