Andrew C. Edmondson and Dr. Brent L. Nielsen, Microbiology and Molecular Biology
DNA recombination is the exchange of segments between homologous molecules of DNA, resulting in expanded genetic diversity. Recombination provides essential genetic variation, but can also cause harmful rearrangements, resulting in genetic disorders in plants, mammals and yeast. The mechanisms and results of these events are still the subject of much research. A variety of evidence supports DNA recombination in plants, but the mechanisms are poorly understood. Researchers have proposed a bacterial RecA-mediated mechanism for DNA recombination in mitochondria based on evidence of an evolutionary relationship between bacteria and mitochondria. However, until recently, plant mitochondria had not been shown to contain any of the proteins needed for this mechanism, including RecA.
Mitochondria are vital components of both mammalian and plant eukaryotic cells, generating essential energy to carry out metabolic processes in cells. Mitochondria also play an important role in cell death, cancer control and are useful in genetic typing. Mitochondrial genomes are separate from the cell’s nuclear genome, but many of their vital proteins are encoded in the nucleus. These genes are translated in the cytoplasm and the proteins are then transported into the mitochondria.
Our laboratory has isolated and identified both a RecA homolog in Arabidposis thaliana which is specifically targeted to mitochondria and is related to a RecA protein homolog in chloroplasts (1) and a putative Single-Stranded DNA Binding protein (SSB) homolog in Arabidopsis thaliana which is specifically imported into mitochondria (2). Both of these proteins are vital to the proposed RecA-mediated recombination mechanism. While RecA serves as the active enzyme in strand exchange, SSB is vital for DNA replication and recombination and can greatly enhance strand exchange. In experiments characterizing RecA-mediated recombination in E. coli, SSB enhanced RecA’s performance by specifically coating single-stranded DNA and preventing the inhibition of RecA, perhaps by directly interacting with the RecA protein (3).
An SSB homolog in Arabidopsis thaliana (At4g11060), which is also predicted to be targeted to mitochondria, was identified using NCBI (www.ncbi.nlm.nih.gov) and TAIR (www.arabidopsis.org) databases. The cDNA clone (SQ130f07F) was obtained from Kazusa DNA Research Institute, Chiba, Japan and found to contain the entire sequence for the putative SSB gene. Importation experiments were conducted in the laboratory to confirm its localization to mitochondria (2).
DNA primers were used to amplify the ssb gene from the cDNA clone, removing the coding region for the mitochondrial transit peptide and allowing the DNA fragment to be cloned into a pCR T7 TA-TOPO expression vector (Invitrogen), adding a six-histidine tag to the N-terminus of the expressed SSB. Plasmid constructs were confirmed by restriction analysis and sequencing. The plasmid was expressed in BLR (DE3) pLysS E. coli cells, grown overnight in Super Broth (BD Biosciences) without IPTG induction. Cells were disrupted by freezing in liquid nitrogen, thawing on ice, and sonicating for 20 minutes with 10 second intermittent bursts every 30 seconds. SSB was partially purified by 40% ammonium sulfate salt precipitation in potassium phosphate buffer. The precipitated proteins were resuspended and then dialyzed overnight in potassium phosphate buffer, using a method adapted from Ehn (4). The protein was then loaded on a nickel column (Invitrogen) and washed overnight with potassium phosphate buffer containing 10 mM Imidazole. Purified SSB was isolated from the column using potassium phosphate buffer with an increasing linear gradient of Imidazole from 10mM to 500 mM. Collected fractions were analyzed by spectrophotometry (BioRad) and run on polyacrylamide SDS-PAGE gels (Invitrogen) and stained with Coomassie Blue to determine a purity of approximately 98% (data not shown). Purified SSB was transferred to storage buffer (50mM Tris-HCl (pH 7.4), 1mM EDTA, 1mM DTT, 0.2M NaCl, 50% (v/V) glycerol) using salt exchange columns (Amersham Biosciences) and stored at -20 °C.
The purified protein’s affinity for single-stranded DNA was assayed using Electrophoretic Mobility Shift assays (Pierce) as described (5). Briefly, increasing concentrations of protein were added to a fixed amount of single-stranded DNA of mixed sequence, labeled with biotin. Protein bound DNA migrates slower than free DNA, resulting in a “shift” when run on a polyacrylamide gel. The DNA is blotted onto nitrocellulose and visualized using streptavidinconjugated horseradish peroxidase (HPR) according to manufacturer’s instructions (Pierce). This shift was compared with the shift produced by E. coli SSB. The mitochondrial SSB produced a similar binding pattern when compared with the E. coli SSB (Figure 1).
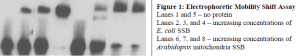
Our results provide evidence to support the currently proposed mechanism of mitochondrial recombination, indicating that the mitochondria SSB homologue binds to single-stranded DNA with similar affinity to E. coli SSB. This SSB homologue is located in the mitochondria, where it could use its single-stranded DNA binding capacities to specifically coat single-stranded DNA and prevent the inhibition of RecA. The SSB homologue’s ability to enhance strand exchange by Arabidopsis mitochondria RecA is currently being assayed.
