Jed Walker
Introduction
Polymer inclusion membranes (PIMs) have been used as an effective means of separation of ions in solution. Due to their mechanical stability, PIMs provide a more effective method of separation than supported liquid membrane (SLM) and bulk liquid membrane (BLM) systems. PIMs also provide higher efficiency, selectivity and ease of use than the previously mentioned systems1,2. The large surface area to volume ratio that is exhibited by PIMs affords them the potential to be used in nuclear and harmful metal waste remediation on an industrial scale.
PIMs are simple in structure. They consist of a polymer that provides mechanical strength, a carrier molecule which effectively binds and transports ions across the membrane, and a plasticizer that provides elasticity and acts as the solvent in which the carrier molecule can diffuse. The carrier molecule acts as a guest-specific host that provides selective membrane permeability for a target species.
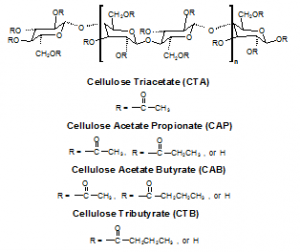
The polymer cellulose triacetate (CTA) has many properties that facilitate it to be used as an effective polymer in the production of PIMs. CTA is easily workable and is a component of many everyday useful products, such as surface coatings, food packaging, filtration systems, and more.3,4 When placed in a neutral solution, CTA membranes are very durable and resistant to breakdown. However, due to hydrolysis of the ester bonds in CTA, it is necessary to find polymers that would hold up in alkaline and acidic solutions.
In an attempt to find a polymer that is more durable and facilitates greater diffusion across the membrane than CTA, I ran experiments that compared the durability and transport of a variety of cellulose-based polymer derivatives. I used cellulose triacetate (CTA), cellulose acetate butyrate (CAB), cellulose tributyrate (CTB), and cellulose acetate (CA).
Objective
The mold setup, on which membranes are made, consists of BYTAC chemical resistant Teflon FEP film backed by a tough support layer coated with adhesive (obtained from Norton Performance Plastics Corporation, 150 Dey Road, Wayne, NJ 07470). The Teflon is adhered to 10” by 6” glass sheets so it can be easily transported and cleaned. Cross-sections from a glass cylinder are prepared with the following dimensions: height 2.5 cm, inner diameter 9.5 cm, and outer diameter 9.8 cm. These glass molds are sealed to the Teflon surfaces with Devcon Epoxy. The membrane apparatus used for both the transport and durability studies consists of two cylindrical glass cells clamped together with the membrane held between them. The solutions in each cell are stirred with glass propellers at a speed of 600 rpm. Each glass cell can hold a volume of 200 mL, and when the membrane is placed between the two cells the membrane surface area exposed to the aqueous phases is 18.86 cm2.
When performing an acid durability study, a 3.0 M solution of HNO3 and a solution of KNO3 is added to the source phase, while in an alkaline durability study 3.0 M KOH will be added to the source phase. Millipore water is added to the receiving phase and 1 mL samples are taken on a regular basis. Potassium (K+) concentration is then measured using atomic emission inductively coupled plasma spectroscopy (ICP), and the results are used to determine the rate of breakdown of each individual polymer membrane. Transport studies require a neutral source phase containing a solution of KNO3 and the rate of diffusion of K+ across the membrane is measured by the same method used in the durability studies.
Conclusion
The disposal of nuclear and harmful metals waste has long been point of interest to environmentalists and society alike. The Hanford site is a perfect example of the need to develop methods of separating hazardous species from basic solutions. At Hanford, sodium hydroxide was added to 177 million gallons of high-level acidic waste in an attempt to precipitate metal species. These highly alkaline wastes (pH 14) still contains such hazardous species as strontium 90.5 Acidic waste solutions are also common since spent fuel rods have been dissolved in acid. We have collaborated with the Idaho National Environmental Engineering Laboratory (INEEL) concerning 0.5-1.5 M H+ wastes containing 90Sr2+ and 137Cs+.. Polymer inclusion membranes present a separation method that could dramatically reduce the amount of harmful waste that is stored in facilities such as Hanford and INEEL by concentrating the harmful ions into a much smaller volume.
References
- Chrisstoffels, L. A. J.; de Jong, F.; Reinhouldt, D. N. Chemical separations with liquid membranes, eds. Bartsch R. A., Way, J. D. ACS Sympossium Series Number 642, ACS, Washington DC, 1996; Chapter 3.a
- Danesi, P. R. (1984-85). Sep. Sci. Technol., 19, 857
- Kirk-Othmer Encyclopedia of Chemical Technology 4th ed. volume 5 1993 editors Kroschwitz, J.I; Howe-Grant M.
- Encyclopedia of Chemical Processing and Design volume 1 1976 editors Mcketta, J. J.; Cunningham, W. A.)
- Levi, B. G. Hanford seeks short- and long-term solutions to it’s legacy of waste, Physics Today March, 17-21 (1992)
