James Bradley Finlay and Dr. Keith A. Crandall, Integrative Biology
Introduction
There has always been a fascination with the great diversity of species that exist on the earth. For my project I chose to investigate this diversity phenomenon by examining the intraspecific relationships between populations of the freshwater crayfish currently considered Cambarus tenebrosus (Hay, 1902) taxonomically (by morphology). This crayfish species is found continuously throughout the central lowlands of the United States (Taylor 1997). C. tenebrosus is unique among other crayfish due to its ability to thrive in a cave environment as well as surface streams and springs. By studying these organisms one can more fully understand the effects that human activity has on two completely different habitats, especially since crayfish are excellent bio-indicators of water quality (Hobbs & Hall, 1974).
Traditional classification relies on morphological features of an organism and such is the case with C. tenebrosus. However, since there is little or no morphological variation between the crayfish found in one habitat versus another, much confusion has arisen with regards to the evolutionary relationships between populations (Taylor, 1997). The main focus of the project was to test three hypotheses that are central to the assignment of species status and population structure. The objectives of this study were to (1) determine if C. tenebrosus is a single species forming a monophyletic group when compared to other species of Cambarus, (2) establish whether C. tenebrous shows sub-divisions at the intraspecific species level (within the species) and (3) test if there is a genetic association between the two habitats the crayfish occupies (cave and surface). Uncovering a species’ phylogenetic history (history of organismal relationships) is key to its protection and conservation (Moritz, 2002).
Materials and Methods
Whole specimens or tissue samples were collected by Jen Buhay (Brigham Young University doctoral student), with approximately 90% of the samples obtained from caves, with the remaining 10% being taken from streams. In most cases non-destructive methods of sampling were used by only removing a leg and returning the individual to the site of capture. For morphological analysis, voucher crayfish were also collected. Each specimen or leg sample was given a unique identification number to coordinate with the latitude and longitude measurements taken by a GPS device.
The DNA was extracted from either the leg or gill tissues of the specimens using a cell lysis protocol (Crandall et al. 1999). The polymerase chain reaction (PCR) was employed to amplify the 16S and COI mitochondrial genes. The cleaned PCR products were sequenced using the ABI Big-Dye Ready-Reaction kit and the sequences were determined using the 3730 XL automated sequencer. The 16S mitochondrial gene was sequenced for all samples because it has shown to be capable of detecting intraspecific divergence (Fetzner and Crandall 2003). For those 16S haplotypes that were unique, the mitochondrial gene CO1 (cytochrome oxidase 1) was sequenced for higher order comparison among other species of Cambarus.
The sequence data obtained from the DNA Sequencing Center was edited using Sequencher 4.2 (Gene Codes Corporation) then aligned by eye using MacClade 4.05 OS X (Madison and Madison 2000). The ‘best-fit’ model of nucleotide evolution was determined using Modeltest 3.06 (Posada and Crandall, 1998). Testing whether C. tenebrosus forms a monophyletic group was accomplished using two statistical approaches, a Bayesian analysis (Huelsenbeck et al. 2001) with the program MrBayes 3.0B4 (Huelsenbeck and Ronquist 2001) and a Maximum Likelihood approach (ML) using PAUP* (Swofford, 2002).
Results
This study is not yet finished but will be completed by early next year (2005). There are only a few crayfish tissues that remain to be sequenced from a recent collection trip. The data set thus far consists of 181 sequences from 4 different states. The Bayesian phylogeny (Fig. 1) and map (Fig. 2) with correlating colors are shown below. A gene network has already been constructed (not shown) using the 16S data set. This network will be used in a Nested Clade Analysis (NCA) which can disentangle historical inferences from geographic and genetic patterns (Templeton, 1998). There are distinct groupings of populations seen in the Bayesian tree which partially correspond to the dots on the map. It is evident that there is a rich population history associated with this animal which will be estimated empirically. Also I am working on choosing other members of the genus Cambarus that will allow better resolution to the tree topologies.
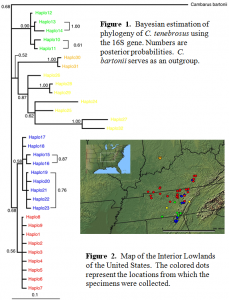
All though this project is not entirely complete, the preliminary results show that there is a great evolutionary “story” to be told. The historical background of the species will be more evident when the NCA has been completed. There are plans to sample other areas of the range of the crayfish (the northern part of Kentucky and the southern part of Indiana) that will be helpful in connecting the populations for which I already have data.
References
- Crandall, K.A., Fetzner, J.W., Lawler, S.H., Kinnersley, M., and Austin, C.M. (1999). Phylogenetic relationships among the Australian and New Zealand genera of freshwater crayfishes. Australian Journal of Zoology 47, 199-214
- Fetzner, J.W., Crandall, K.A. (2003). Linear habitats and the nested clade analysis: an empirical evaluation of geographic versus river distances using an Ozark crayfish (Decapoda: Cambaridae). Evolution 57(9), 2101-2118
- Huelsenbeck, J. P. and Ronquist, F. (2001). MrBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17, 754-755.
- Huelsenbeck, J.P., Ronquist, F., Nielsen, R., and Bollback, J.P. (2001). Bayesian inference of phylogeny and its impact on evolutionary biology. Science 294: 2310-2314
- Hobbs, H. H., Jr & Hall, E. T., Jr (1974). Crayfishes (Decapoda: Astacidae). In Pollution ecology of freshwater invertebrates: 195–241. Hart, C. W., Jr & Fuller, S. L. H. (Eds). New York, NY: Academic Press.
- Madison, D.R. and W.P. Madison. (2000). MacClade 4. Analysis of phylogeny and character evolution. Sinauer Associates, Sunderland, Massachusetts.
- Moritz, C. 2002. Strategies to Protect Biological Diversity and the Evolutionary Processes that Sustain It. Syst. Biol. 51:238-254.
- Posada, D. and K.A. Crandall. (1998). Modeltest: testing the model of DNA substitution. Bioinformatics 14, 817-818.
- Swofford, D.L. (2002). PAUP* Phylogenetic Analysis Using Parsimony (*and other methods), Sinauer Associates, Sunderland, MA.
- Taylor, C.A. (1997). Taxonomic status of members of the subgenus Erebicambarus, genus Cambarus (Decapoda:Cambaridae), east of the Mississippi River, Jor. Of Crustacean Biology, 17(2), 352-360
- Templeton, A.R. (1998). Nested clade analyses of phylogeographic data: testing hypotheses about gene flow and population history. Molecular Ecology 7, 381-397
