David G. Evans and Dr. Matt A. Peterson, Biology and Agriculture
Until only very recently it has been the common practice of most pharmaceutical manufacturers and manufacturers of agrochemicals such as herbicides and insecticides to market chiral compounds as mixtures of enantiomers. This practice has been driven by market forces due to the fact that it is much more cost efficient to market chiral compounds as mixtures rather than invest the resources needed to separate them. Tragic cases have shown that there is indeed a need for complete separation of enantiomers and their separate pre-clinical and clinical evaluation.
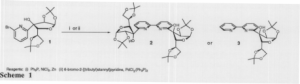
An approach to problems associated with mixed enantiomers involves synthesizing chiral molecules in such a way that one of the enantiomers is favored over the other. This kind of synthesis is referred to as enantioselective. Enantioselective synthesis eliminates the need for separation of enantiomers or at the very least greatly diminishes the cost involved in such separations. My research focuses on development of new ways to synthesize biologically relevant enantiomers in an enantioselective fashion. Emphasis during the past six months has been placed on the use of chiral bidentate ligands 2 and 3 (Scheme 1) as ligands for enantioselective transition-metal-mediated catalysis.
I have successfully produced compound 1 in good yield on a multi-gram scale. It plays a pivotal role in our area of research, because from it we can produce two bipyridines (compounds 2 and 3). Compound 1 is synthesized by producing diacetone glucose, and then oxidizing it to its ketone. I then add 2, 6-dibromopyridine by means of lithium-halogen exchange (Scheme 2).
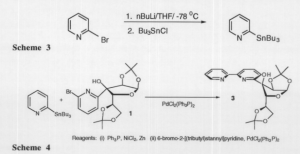
Synthesis of compound 3 proved to be quite capricious. Tributyltin pyridine was produced with good yield (Scheme 3). With compound 3 in hand, my emphasis turned to coupling it to compound 1 with bis-triphenylphosphine palladium chloride as the catalyst (Scheme 4).
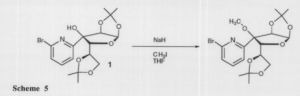
Because of a problem of tin coordination with the hydroxyl group on compound 1, we changed our direction to methylation of compound 1, followed by addition of the tributyltin pyridine (Scheme 5). The methyl group protects the compound from tin coordination.
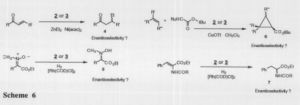
I have successfully synthesized two new ligands. The key step in these syntheses is the highly selective addition of 2-bromo-6-lithiopyridine to diacetone glucose. These ligands will be collectively investigated for their ability to influence enantioselectivity in the synthesis of a number of biologically important compounds, as illustrated below (scheme 6).