Keith Wells and Dr. Kim O’Neill, Microbiology and Molecular Biology
It has been estimated that over 560, 000 Americans will die of cancer this year, over 1,500 a day. Currently, there is much research performed to understand how cancer works, how to prevent it, and how to treat it. Cancer encompasses a multitude of diseases coming from nearly all tissue types in the body and from all age groups. It is a problem which plagues all countries and cultures.
On a molecular and cellular level, cancer is uncontrolled growth and deregulated death in cells of the body. Mutations and other abnormalities of the genome, accumulating over the course of a lifetime, lead to cellular instability. Normally, the body keeps these renegade cells in check, destroying those which show precancerous signs. However, the mutations which cause the cancer cell to begin to proliferate can also subdue the body’s defense. It is not currently known how the cancer cells evade the immune system. It has recently been suggested that through cell-to-cell signaling, cancer cells may even recruit cells of the immune system to help them grow and spread. The spread of cancer cells is called metastasis.
The purpose of my research is to better understand which portions of the immune system interact with cancer and if this interaction hinders or helps. To do this, our lab has utilized an in vitro mouse model of melanoma. Murine immune systems are the most similar to human of all the laboratory animals used at BYU. Through humane techniques approved by ORCA’s safe care and handling guidelines (IACUC), we can model how melanoma cells interact with immune cells and metastasize throughout the body. The mice that we use are called Mafia mice. Mafia stands for macrophage fas-induced apoptosis. These mice have special macrophages: they can be eliminated though the addition of a synthetic drug.
Macrophages make up an important part of the immune system. Monocytes, circulating white blood cells which eat debris and foreign particles in the blood, enter tissues and become macrophages. They are present in nearly every tissue in the body. In the presence of a wound or infection, they are particularly active. In many cancer types, including melanoma, macrophages make up a large proportion of cancerous tissue. It is unclear all of the roles macrophages play at the site of a tumor.
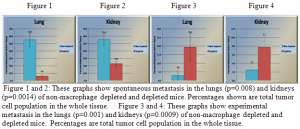
Previously in the lab, a team of students, including myself, showed that macrophages are directly linked to metastasis. When macrophages are depleted from a Mafia mouse prior to the introduction of melanoma, then the melanoma cells did not spread to the lungs and kidneys as well. These two organs are the usual sites of distant metastasis. This type of metastasis is called spontaneous metastasis. It occurs when tumor cells detach from their original position and find a new location after traveling through the blood stream. From these experiments, we see that macrophages are critical to the spread of tumor cells.
For my project, I wanted to continue to understand the role that macrophages play in the metastatic process. This led me to look at different forms of metastasis in mouse models. As described above, the first and most common is spontaneous metastasis. There are two other recognized forms of metastasis in mouse models which are important to understanding the whole picture of a migrating tumor cell. The first is called experimental metastasis. It involves injecting tumor cells directly into the blood stream through the tail vein of a mouse. Thus, experimental metastasis removes the obstacle for the tumor cells of locating and breaching into the blood stream. The third type of metastasis is called bony metastasis. Tumor cells are injected directly into the heart in order to bypass the lungs which trap most tumor cells from the blood stream. All three give information about the varying aspects of metastasis.
For my experiment, I chose to obtain results of experimental metastasis under the same conditions we had previously worked. Then I could compare the results from our work with spontaneous metastasis and get a better understanding of the role that macrophages play in the process of metastasis. In the future, it will be interesting to look into bony metastasis, but currently, no one at BYU has the training required to perform and teach the procedure.
The results of my experiments are found in Figures 1-4. Figures 1 and 2 show spontaneous metastasis in both the control and macrophage-depleted mice. These two figures were the results of previous work I mentioned earlier. As seen, by depleting, or removing macrophages from the tumor environment, the level of metastasizing tumor cells significantly drops. Thus, macrophages are needed by the tumor cells to metastasize. In Figures 3 and 4, we see the same conditions, only with experimental metastasis. Here we see the opposite trend. In these data, macrophages are hindering the progress of tumor cells. By looking at both sets of data, it becomes evident of the multifaceted role of macrophages in tumor progression and metastasis.
One setback which occurred late in my experiments will prohibit publishing this work while at BYU. On the last data set (each takes about one month), the cellular staining failed. I will leave my work to my mentor and hope that the fellow students I trained will finish out my experiments. The experience I had working in the lab has been a tremendous advantage to me. It has not only helped me develop better critical thinking skills, but has been an intense area of interest as I interviewed for professional schools.