Christopher D. Rice and Dr. William McCleary, Molecular and Microbiology
Phosphate is a necessary chemical compound for life at any level. Organisms have developed different means of obtaining phosphate from their environments and maintaining an intracellular phosphate homeostasis. The creation and maintenance of such a homeostasis at the very least requires mechanisms for sensing phosphate levels within and without the cell and phosphate transporters which can be regulated accordingly. The protein PhoU is involved in this phosphate response regulation. While PhoU is found in Escherichia coli, a very broad range of bacteria contain homologues with several closely conserved regions suggesting not only that PhoU-like proteins are important for many microorganisms in controlling intracellular phosphate levels but also that they likely share a similar mechanism of action at the molecular level. PhoU has been studied since the mid 70’s and is known to play a vital role in the phosphate sensing/signaling interactions of the cell and, while perhaps there is a basic understanding of at least part of its’ role in these interactions, the big question remains how? In our efforts described here, we attempted to identify mutant PhoU proteins in hopes of shedding light on its’ molecular mechanism of action.
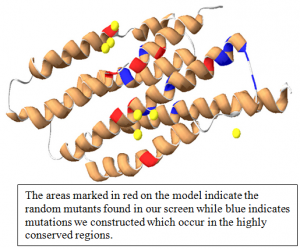
In E. coli there exists a high affinity phosphate transporter called the PstSCAB transporter. This is an active transport system, more specifically an ATP Binding Cassette (ABC) transporter, which is activated when environmental phosphate levels are low. When environmental phosphate levels are high, PhoU acts as its’ negative regulator and represses the expression of the PstSCAB transporter as well as many other genes in the Phosphate (Pho) regulon (a group of genes under the same regulation). In order to understand better what PhoU was doing and what parts of the protein were “important” for those interactions, we wished to study PhoU mutants which displayed decreased levels or function. In high phosphate media, a decreased level of function would manifest itself as increased expression of PstSCAB genes and the entire Pho regulon, meaning the PhoU mutant was not negatively regulating as it should. Utilizing this principle, a large genetic screen identified several such mutants. We sequenced these mutant genes and identified were in the protein these mutations were located. Using the Swiss PDB Viewer I created a 3D model of PhoU and mapped the mutations found in the screen. We expected to find a greater correlation between the mapping sites of our randomly generated mutants and sites previously identified as highly conserved.
We then performed Akaline Phosphatase Assays on both the randomly generated mutants and the deliberate (site-directed) mutants in order to roughly quantify the effects these mutations had on PhoU function. Akaline Phosphatase is encoded in the before mentioned Pho regulon and its’ presence/abundance is easily detected, thus it acts as a reporter gene for the entire regulon. The results from these assays showed that most of the mutations, whether random or site-directed, only slightly decreased function as compared to the normal PhoU protein. This was somewhat puzzling and led us to propose that perhaps the PhoU protein has functional redundancy built into it’s structure. In other words, perhaps there are two regions of the protein which do the same thing and so a mutation which disabled one region would still leave the other functioning normally.
While conducting these experiments we noticed that the strain in which we were harboring our PhoU mutant genes (this strain completely lacked a chromosomal copy of phoU, plasmid versions of the mutant genes were used for reasons not explained here) behaved strangely. When this strain was completely lacking any version of phoU it exhibited a normal growth rate but a decreased growth yield. This decreased growth yield was exacerbated in high phosphate media.. We wondered whether an over accumulation of phosphate was poisoning the strain and was responsible for this decreased growth yield. Also important about this strain is the fact that we had decoupled the ability of PhoU to up regulate expression of the PstSCAB transporter. Therefore to explain why this over accumulation of phosphate might be happening when there should not have been increased amounts of the PstSCAB transporter we proposed that perhaps PhoU is responsible not only for the abundance of the PstSCAB transporter in normal cells but also controls its’ activity. In other words, while the absence of PhoU in our strain could not be responsible for increased PstSCAB levels (and thus too much phosphate uptake) we wondered if PhoU is still needed to regulate the transporter activity, ie inhibit its’ activity. Phosphate uptake assays were performed which showed that indeed our strain was taking up more than normal amounts of phosphate. This strongly substantiates our claim and gives evidence for an additional function of PhoU.
This was a wonderful experience for me and I honestly consider it one of my greatest academic accomplishments. A manuscript has been prepared and submitted to the Journal of Applied and Environmental Microbiology and we anticipate it being published there in the near future. My mentor, Dr. McCleary, was a wonderful and patient teacher and he really helped me understand this material at a whole new level. This experience has also excited me about the prospect of doing research in the future. Even more importantly, I gained a greater appreciation for our Maker and his hand in the creation of all forms of life. The complexity of even a “simple” bacterium is to me one more testimony of his wisdom and knowledge and it was a privilege for me to be able to learn so much from the small and meek things of the earth.
