Brandon Forbes
Neurons communicate to other cells by releasing neurotransmitter through a process called exocytosis. Exocytosis of neurotransmitter containing vesicles to their plasma membranes is via the SNARE protein complex. Studies that have examined the SNARE protein complex, using an in vitro lipid bilayer model, have thus far not examined the effects of cholesterol on the SNARE driven fusion. As a research hypothesis, I expect that cholesterol will have a positive effect on the rate of SNARE driven fusion events.
The cholesterol could provide a more fluid membrane allowing the SNARE complex to cause fusion events to take place by reducing the amount of energy the complex needs to come up with. Understanding the role of cholesterol can lead to a greater understanding of how the SNARE complex directs vesicular fusion. In order to test this hypothesis, I will use a lipid bilayer fusion assay developed by Dr. Dixon Woodbury at Brigham Young University.
An artificial lipid bilayer was made separating two chambers of saline buffer, and vesicles were allowed to fuse with cholesterol in the target membrane. Along with this group of cholesterol treated target membranes, my fellow bilayer team members and I also ran experiments allowing vesicles to fuse to a target membrane that does not contain cholesterol. The target membrane consisted of the lipids PE and PC mixed in a 7:3 ratio. This composition of lipids was in its fluid phase at room temperature and simulated normal neuronal conditions. Vesicles were allowed to fuse spontaneously with this fluid membrane with and without 40mol% cholesterol. The fusion rates were also compared to establish the effect of cholesterol on the fusion of native vesicles. The lipids for the planar membrane were stored dissolved in chloroform in a 10 mg per mL concentration. The lipids were stored at -20 degrees C. In preparation for use, the lipids were warmed to room temperature to prevent condensation. The lipids were dried under a stream of nitrogen gas to prevent their oxidation. The dried lipids were then mixed with decane to arrive at a 20 mg per mL concentration. This 20 mg per mL concentration were then used to “paint” the planar membrane in the chamber.
The modified synaptic vesicles that have been used in this assay were prepared using a mix of artificial vesicles containing nystatin and ergosterol and native synaptic vesicles purified from rat brains. The native synaptic vesicles provide us with a source of SNAP-25 as well as Syntaxin. The artificial vesicles were protein free and contain nystatin and ergasterol. The nystatin and ergosterol enable detection of exocytosis by forming channels in the artificial vesicles that allow ions to pass through the membrane, allowing us to see an electrical current when the vesicles fuses. The synaptic vesicles from the rat contained all the native proteins that the rat’s neurons attach to the vesicle during development. In order to fuse the artificially made vesicles containing channels with the rat synaptic vesicles, the two populations of vesicles were mixed together. The mixture of artificial and synaptic vesicles were frozen to rupture the vesicles, allowed to thaw so that the vesicular lipids can interact, and then sonicated in order to cause the lipid mixture to form new vesicles. The resulting vesicles contained membrane from both the synaptic vesicle and the artificial vesicle populations creating a modified synaptic vesicle. By fusing these artificially made vesicles containing channels with the native rat neuronal vesicles, vesicles were obtained that still had the normal behavior of synaptic proteins as well as channels that allow current to pass through them. As these modified synaptic vesicles fused to our artificial membrane the channels in the vesicle allowed electric current in the form of ions to pass through. This allowed us to observe fusions as literal spikes on a graph depicting current in pA versus time and directly count each individual fusion event.
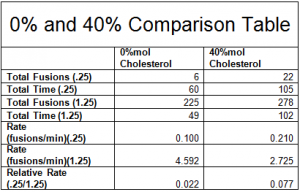
One way to analyze our data is to look at the totals of the fusion rates across all of our experiments. By adding the fusions and time of each experimental condition, we can compare the relative fusion rates across all experiments. Please note from the table below that the total relative fusion rate of the 40%mol cholesterol is 3.5 times more than the 0%mol cholesterol – suggesting a significant effect.
The two sample t-test is designed to look at the difference of two separate samples of data. Our results indicate that we have significant results at an alpha value of .1 with a p-value of .0723. As a result, I will conclude that cholesterol does have an effect on SNARE driven fusion rates. This is a reasonable conclusion as there is only a 7.23% chance of getting this data if the null hypothesis, cholesterol does not have an effect, is correct. Due to the variability of lipid bilayer experiments, more experiments will be conducted in the future in order to provide a more reliable p-value.
![]()
