Lauren Clough and Dr. Jeffery R. Barrow, Physiology and Developmental Biology
Introduction
Vertebrate embryonic limb development is a complex process that involves many genes and processes. Patterning takes place along three axes, including the proximal-distal axis of the limb. The Apical Ectodermal Ridge (AER) helps to guide development along the proximal-distal axis, but the genetic and cellular mechanisms whereby it performs its roles are not well understood. This study attempted to discover whether the AER is able to recruit limb cells through oriented cell division through experiments with surgical manipulation of the AER in chicks and genetic manipulation in mice. By using cellular markers to determine apoptotic and mitotic indices, we have collected data that will help form a foundation for future studies in this project and will ultimately help reveal the role of the AER in the development of the vertebrate limb.
Despite much research, the role of the AER in outgrowth and patterning of the limbs is not understood. Scientists differ in their theories of how the AER communicates with other cells, and how it is able to form the bones and tissues of the limb in a specific and ordered proximal-distal manner. The prevailing model, known as the progress-zone model, proposes that the length of time that the deeper cells spend next to the AER determines the type of skeletal element that will form. Cells that are within the signaling range of the AER, termed the progress zone, proliferate. Only a finite number of cells can remain within the progress zone and cells that are pushed out early become proximal structures, while cells that remain in the zone longer become distal structures. Another group has proposed that the AER simply keeps the cells within the signaling range alive. Without the AER, according to this model, the cells would not survive and would undergo apoptosis instead.
Our model hypothesizes that the role of the AER is to recruit cells towards it. Consistent with this hypothesis, we have found that the daughter cells of labeled limb cells within the signaling range will divide towards intact regions of the AER. This recruitment is possible through directional cell movements and cell divisions. This project helped to investigate these three models and discover why, when portions of the AER are removed, depressions in the limb bud form.
According to the progress zone model, depressions would form because the region beneath the removed AER would not proliferate. According to the other proposed model, depressions would form because the cells beneath the removed AER would undergo apoptosis and not survive. In our recruitment model, however, cells beneath both intact AER and removed AER would not exhibit any difference in apoptosis or proliferation when compared to each other.
Experiments and Results
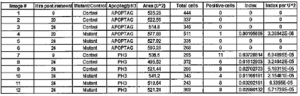
To investigate the hypothesis, I did experiments using both the chick and the mouse as model organisms. I surgically removed portions of the AER in chicks and attempted to genetically remove portions of the AER in mice. I then analyzed apoptosis in the chick limbs by using immunohistochemistry to identify apoptotic and proliferative cells. I counted positive cells in images obtained with a confocal microscope and divided them by the total number of cells in the field counted to obtain apoptotic and proliferation indices. These numbers were then compared in regions that were adjacent to removed AER and regions adjacent to intact AER. The indices do not exhibit significant differences between the two types of regions (see table 1).
Discussion
Because of troubles with experiments, we spent many hours on these experiments and repeated them countless times in order to generate data. We were unable to obtain any data from our genetic crosses in mice as the gene of interest was lost last summer and our colony of mice is now wild-type. Our chick experiments went well, except we had trouble until the end in getting our immunohistochemistry to work. Due to the difficulties in our project, I was unable to generate statistically significant data. However, this preliminary data shows little difference between the indices obtained and may support our model. I was also able to help form a framework for future work on this project that will be instrumental in allowing future students to generate enough data to elucidate information on the pattern of development in the vertebrate limb.
This study has added insight into research investigating limb development. As research progresses in this area, more knowledge will be gained about the mechanisms of birth defect formation and could have a significant impact for good in the lives of humans. Because limb deformities and other birth defects can present challenges throughout an individual’s life, these studies will help to eventually explore ways in which we can prevent defects after genetic screening in parents or gene therapy techniques that could be developed. Before this is possible, however, scientists must fully understand the genes, signal cascades, and morphological changes that occur during embryonic development.
Table 1
Apoptotic and Mitotic Indices. Apoptosis is not seen in the mesenchyme and proliferation is not significantly different between control and modified limbs. When counting positive cells for apoptosis in the images, only one positive cell was noted in a modified limb. This result is not significant and could be due to other factors. In the proliferation studies, the proliferative index of both modified limbs and control limbs were very close and show no remarkable difference between the two. This data does not support previously proposed models but does agree with the recruitment model.