Douglas Bennion and Dr. Jeff Edwards, Physiology and Developmental Biology
Learning and memory are phenomena that are made possible via physical changes at neuronal synapses in the brain, a phenomenon known as synaptic plasticity. Because synaptic plasticity affects physical changes in the brain in response to external stimuli, it is suspected of playing an essential role in strengthening or weakening the neural pathways regulating short-term learning and memory within the hippocampus. Dysfunctions in synaptic plasticity contribute to such diseases and disorders as Alzheimer’s Disease, Parkinson’s Disease, and retrograde amnesia.1 By further clarifying the mechanism of synaptic plasticity, in which TRPV1 is suspected of participating, I have tried to fill a research gap that may bring science and medicine a step closer to producing treatments for such debilitating illnesses. Certain receptors that mediate synaptic plasticity have been extensively studied, specifically the NMDA and other glutamate receptors, while the TRPV1 receptor, which is likely to modulate plasticity, has received far less attention. My specific area of research has explored the effect of TRPV1 activation and blockade in modulating LTP and among the pyramidal cells of the CA1 region of the hippocampus.
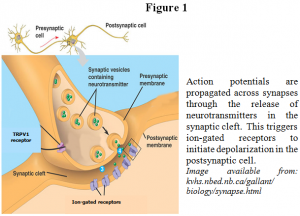
Plasticity is the ability of the brain to physically change in response to experience. Thus, synaptic plasticity involves the physical changes that occur at synapses, the locations where two neurons communicate, in response to frequent and repeated signals from the brain. Two important types of synaptic plasticity, long-term potentiation (LTP) and long-term depression (LTD), serve many functions during development and during all forms of experience-dependent plasticity, including learning and memory.2 In a basic sense, LTP and LTD enable the brain to physically change in response to experience. The brain receives, interprets, and reacts to sensory information via small electrical impulses propagated along neuronal axons and across synapses. At the synapse, the presynaptic cell releases neurotransmitter into the synaptic cleft, which are then received by ion-gated receptors in the postsynaptic cell (see Figure 1). In some cases, the binding of neurotransmitters on the postsynaptic cell can begin a second messenger cascade that may result in retrograde messengers traveling back to bind receptors, such as TRPV1 receptors, located on the presynaptic terminal. In the case of LTD, the number and activity of various receptors found at a specific synapse is decreased, thus depressing or silencing that synapse and making it more difficult to propogate electrical signals, known as action potentials, along that pathway. During LTP, the opposite is true; existing receptors are further activated and new receptors are inserted into the membrane, thus potentiating the synapse and facilitating signaling along the pathway. This shifted potentiation can be maintained for a period of minutes, hours, days, or even years.3 By altering the ease of using certain pathways, the brain is able to “remember” certain pathways over others, thus making learning and memory possible. The role of TRPV1 in the mechanisms of LTP and LTD the been the area of interest in my research.
Experiments were performed in hippocampal rat brain slices that were perfused with artificial cerebral spinal fluid at 28-32C. The excitatory post-synaptic potentials (EPSPs – the electrical response of hundreds of hippocampal neurons) generated in response to electrical stimulus was measured using a glass recording electrode placed 400-600 m from the bipolar stimulating electrode. Following electrical conditioning by either high frequency stimulus (electrical stimulus of approximately 1 mA at 100Hz twice) or theta burst (two bursts of 5 pulses at 100 Hz repeated at 200 sec intervals ten times) pyramidal cells exhibit LTP. Using Capsaicin and Capsazepine (specific TRPV1 agonist and antagonist, respectively) in conjunction with picrotoxin (specific inhibitory-GABAA antagonist) allowed comparison of the LTP response of pyramidal cells with and without TRPV1 receptor activity. Including a set of picrotoxin experiments allowed comparison of experiments with and without the inhibitory effect of GABAergic interneurons. Previous research has explored the LTD-inducing effect of TRPV1 activation on interneurons.4 I hypothesize that activating TRPV1 receptors using Capsaicin will enhance pyramidal cell LTP and that this enhancement will be reversed in the presence of TRPV1 antagonist Capsazepine.
Researchers have recently published several articles related to TRPV1 modulation of synaptic plasticity. They have demonstrated that TRPV1 receptors mediate LTD of the hippocampal CA1 stratum radiatum interneurons.4 In addition, TRPV1 knock out mice were shown to express smaller CA1 LTP than wild type mice,5 and application of high doses of TRPV1 agonist capsaicin increased CA1 LTP in young Wistar rats,6 however the mechanism mediating this effect is not known. My research in Dr. Edwards lab has sought to define a mechanism for this TRPV1 mediated increase in CA1 pyramidal cell LTP. Based on the data from our current investigation in Dr. Edwards’ lab, we have not rejected our hypothesis that TRPV1 mediates LTP via the inhibitory interneurons. Our data indicates that LTP enhancement among CA1 pyramidal cells during TRPV1 seems to be mediated by an inhibitory circuit involving the inhibitory interneurons. I was able to present our findings at our recent poster presentation at Neuroscience 2008 in Washington D.C.7
References
- Brun, VH, et al. J Neuroscience, 21(1):356-362.
- Malenka, R, Bear, M. Neuron 44:5-21.
- Santiago ramon y cajal: biography. Available from: http://nobelprize.org/ nobel_prizes/medicine/ laureates/1906/cajal-bio.html
- Gibson HE, Edwards JG, Kauer JA, et al. Neuron 57(5): 746–59. Marsch R, Wotjak C., et al. J Neuro. 27(4): 832-839.
- Li H, Xu L., et al. Biol. Psychiatry. 64(4): 286-292.
- Thanks to Jensen T, Couch J, Castle M, Daniel S, Nelson B & Edwards JG for their collaboration.