Dr. John Bell, Department of Physiology & Developmental Biology
Abstract of original proposal
Secretory phospholipase A2 (sPLA2) binds to and hydrolyzes cell membranes. It is important in inflammatory responses and diseases including septic shock, atherosclerosis, and cancer. Normally, cells resist the enzyme’s action, but they become susceptible early during the process of either biochemically-programmed or of traumatic cell death. A recent discovery demonstrated that the activity of different isozymes of sPLA2 depends on the type of cell death involved. Understanding the relationship between this specificity and physiological roles of these isozymes requires elucidation of the relevant molecular mechanisms. This project will test the hypothesis that the relative levels of four membrane biophysical parameters determine the activity of each isozyme to hydrolyze dying cells: 1) binding of merocyanine 540, 2) membrane solvation, 3) permeability of propidium iodide, and 4) phosphatidylserine exposure on the outer membrane face. The hypothesis will be examined by using a variety of agents to induce a spectrum of cell death modes ranging from apoptosis to necrotic death caused by oxidative stress. The activity of various sPLA2 isozymes to hydrolyze the cell membrane and the levels of the four parameters will be assayed for each type of cell death. A quantitative global model that combines the information from these assays to predict each isozyme’s specificity toward the mode of cell death will then be generated and evaluated. The primary method employed to asses membrane physical properties will be fluorescence spectroscopy. Subtle changes in membrane permeability to propidium iodide will be quantified by flow cytometry. Membrane hydrolysis will be assayed with a fluorescent fatty acid binding protein. In summary, this project will help guide a novel direction in sPLA2 research relating the biophysics of cell membranes to enzyme activity in the physiological and pathological setting of cell death.
Hypothesis tested
We hypothesize that cell membrane susceptibility to sPLA2 is governed by a balance of four phenomenological physical parameters: 1) the capacity of the membrane to bind the probe merocyanine 540 (MC540), 2) the degree of membrane solvation, 3) the level of membrane permeability to vital stains, 4) the amount of phosphatidylserine on the membrane surface. The membrane becomes more vulnerable to attack when the values of these parameters increase. Specificity is determined by the level of sensitivity of individual sPLA2 isoforms to each parameter and by the relative prominence of each during the various modes of cell death.
Specific aim
Determine the basis for differential susceptibility of lymphoma cells to hydrolysis by human sPLA2 isoforms depending on the mode of cell death. Experiments will focus on the four parameters in the general hypothesis using fluorescence studies with membrane probes (steady-state spectroscopy, microscopy, and flow cytometry) and enzyme activity assay. Snake venom and human sPLA2 isoforms will be used such as groups X (hGX), V (hGV), and IIa (hGIIa). Seven death stimuli that induce death by different modes spanning the continuum from hormone-induced apoptosis to catastrophic accidental death will be compared.
General evaluation
The project went great. The necessary experiments have been completed and five papers reporting the results are in process. One has been reviewed and is now in process of minor revision for resubmission and likely publication. The other three articles are in preparation for submission at the end of 2011 or early in 2012. Finally, the results provided important data for submission of a grant proposal to NIH in October, 2011.
Results
The general hypothesis was evaluated for death by the following means: calcium ionophore, endoplasmic reticulum stress (thapsigargin), glucocorticoid-stimulated death, DNA damage (daunorubicin), block of DNA synthesis (methotrexate), block of DNA transcription (actinomycin D), and block of mitosis (paclitaxel). Four important observations were made.
- Six of the seven death stimuli behaved very similar to each other. Each resulted in:
- greatly enhanced binding of MC540
- a subpopulation of cells with modest permeability to propidium iodide, a vital stain
- increased membrane solvation as revealed by the fluorescent probe patman
- substantial exposure of phosphatidylserine
- The death stimulus that behaved differently was calcium ionophore. It caused all of the results described above except for the modest permeability to propidium iodide.
- We discovered through experiments with new fluorescent probes, patmanand trimethylammonium diphenylhexatriene that the enhanced binding of MC540 and the increased solvation are really manifestations of the same phenomenon, diminished order and packing of lipids in the cell membrane.
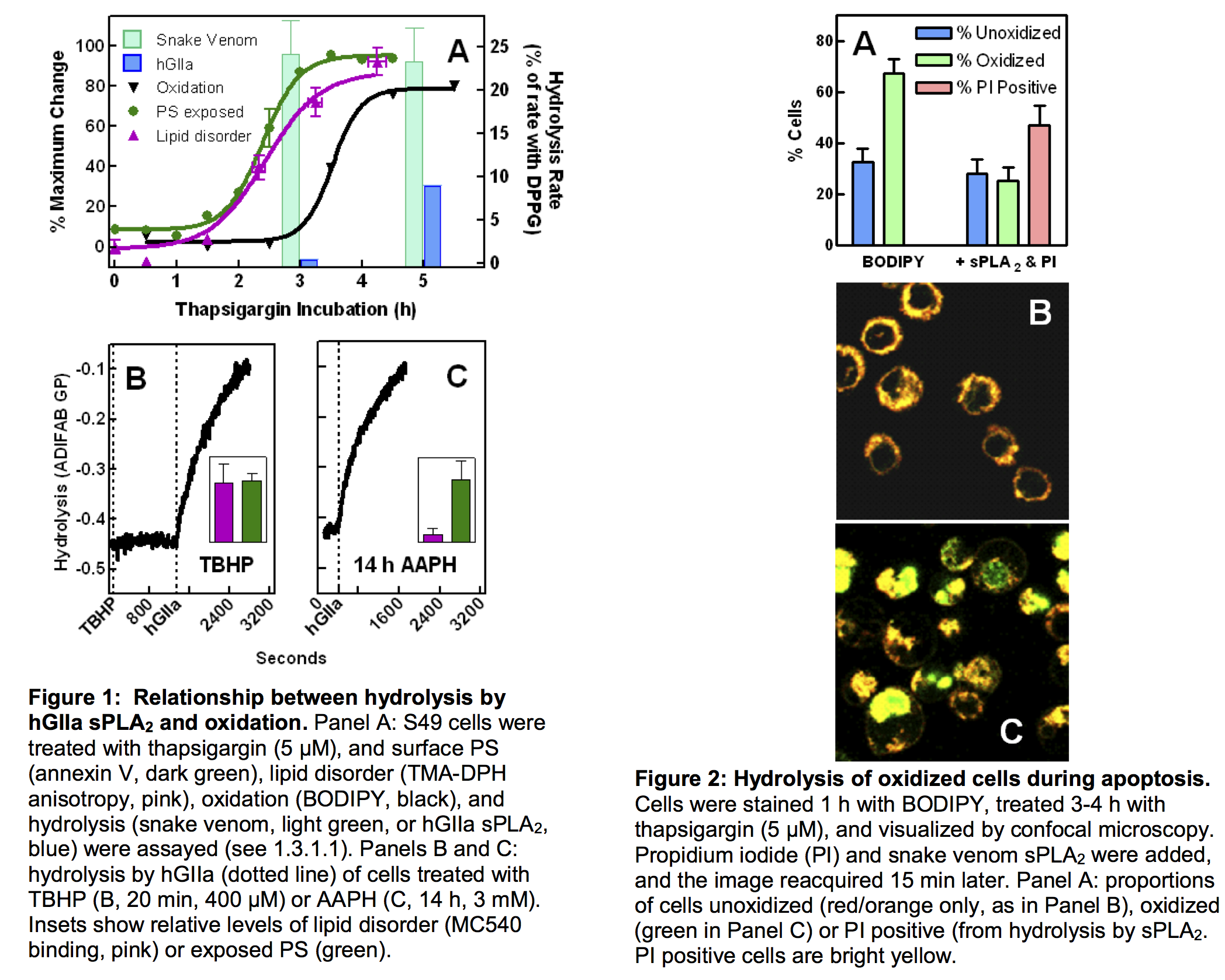
- We discovered that the modest permeability to propidium iodide is actually oxidation of lipids in the cell membrane. This oxidation is distinguishable from the disordering of membrane lipids and exposure of phosphatidylserine. However, it is responsible for governing the hydrolysis of the membrane by the group IIa isoform of sPLA2 (hGIIa). Figure 1A shows the effects of oxidation occurred later than other membrane changes for cells treated with thapsigargin. For example, at 3 h, the reduction in lipid order and PS exposure both approached completion while lipid oxidation was still at very low levels. At 3h, the activity of snake venom was at its highest level, and the activity of hGIIa was much lower (nearly at 0 on the graph). However, at 5 h, when the oxidation potential peaked, hGIIa activity was 25-fold greater. However, in ionophore-stimulated cells, which are not vulnerable to hGIIa, little evidence for oxidation was observed. Furthermore, tert- butyl-hydroperoxide (TBHP, Fig. 1B) and 2,2′-azobis-2-methyl-propan-imidamide, dihydro-chloride (AAPH, Fig. 1C), two agents which oxidize membranes directly, both stimulated hydrolysis by hGIIa to levels 20-100 times greater than any observed previously. Figure 2 provides visual evidence that cells with increased oxidation are attacked by sPLA2. Confocal images of S49 cells stained with C11- BODIPY, a probe of oxidative potential, identified cells oxidized during apoptosis. Co-staining with propidium iodide followed by addition of sPLA2 indicated which cells were vulnerable to the enzyme (i.e. those with yellow-stained nuclei). Cells with oxidative potential were attacked by sPLA2 (i.e. reduced green bar in Fig. 2A). The insets of Figs. 1B and C revealed another unexpected result: although TBHP and AAPH both induced exposure of PS and high activity of hGIIa, only TBHP produced a change in membrane order. Thus, even though membrane disordering was critical in earlier studies (1,5,32,39), it may not be required when the membrane is highly oxidized.
- Weverifiedthatfragmentation(blebbing)oflymphomacellsduringcelldeath also depends on some of these membrane properties (phosphatidylserine exposure and membrane lipid order.
The conclusion reached from these data is that we can now identify very clearly three specific physical events that seem capable of explaining the level of susceptibility of cell membranes to sPLA2: the level of membrane order, the amount of phosphatidylserine present on the cell surface, and the degree of oxidation of membrane lipids. This conclusion became the basis of our hypothesis for a research proposal to NIH. It also provides the foundation for our next MEG proposal.
Students mentored
The following students were mentored at some level through this award. Graduate student salaries came from other sources available to the PI. Kelly Damm was funded partially through the MEG and partially from a fellowship from the Cancer Research Center.
|
Name |
Salary funded by MEG |
Co-author on paper in revision for re- submission? |
Co-author on one or more papers in preparation?
|
Co-author on one or more abstracts for Biophysical Society Meeting in 2011? |
Co-author on one or more abstracts submitted for Biophysical Society Meeting in 2012? |
|---|---|---|---|---|---|
|
Hannabeth Franchino (grad) |
no |
yes
|
yes |
||
|
Elizabeth Gibbons (grad) |
no
|
yes
|
yes
|
yes
|
|
|
Lauryl Campbell (grad) |
no |
yes |
yes |
||
|
Lynn Anderson (ug) |
yes |
yes |
yes
|
yes |
|
|
Amanda Berbert (ug) |
yes
|
yes
|
yes
|
||
|
Eric Moss (ug) |
no |
yes |
yes |
||
|
Joseph Chen (ug) |
yes
|
yes |
yes
|
||
|
Sarah Franklin (ug) |
yes |
yes |
yes |
||
|
Lyndee Francom (ug) |
yes
|
yes |
yes
|
yes |
|
|
Mikayla Olson (ug) |
no |
yes |
yes |
||
|
Izadora Izidoro (ug) |
yes |
yes |
yes |
yes |
|
|
Kelly Damm (ug) |
partial
|
yes |
yes |
yes |
|
|
Evan Stevens (ug) |
yes |
yes |
yes |
yes |
|
|
Stephanie Melchor (ug) |
yes
|
yes |
yes |
||
|
Allie Vaughn (ug) |
no |
yes |
|||
|
Mara Whitworth (ug) |
yes
|
||||
|
Linea Kemsley (ug) |
no |
yes |
|||
|
Mike Murri (ug) |
no |
Evaluation of mentoring environment
The original proposal was to fund seven undergraduate students. In fact, we were able to provide some level of salary funding to 10. Five additional students also participated for course credit rather than pay. All but two of the students have been involved for multiple terms/semesters, and those two are committed to continuing with us in the future. Consequently, 11 have thus far been involved at a level that justifies co- authorship on one or more publications. Likewise, several assisted with preparing data for presentation at the annual international meeting of the Biophysical Society either in 2011 or on abstracts for the 2012 meeting. Although no undergraduate students were able to attend the meeting in 2011, the 2012 meeting will be in San Diego, and we will be driving a van to accommodate as many of the students as would like to attend. Those who attend will be involved in presenting the data in posters at the meeting. Weekly lab meetings were held throughout the year in which students presented their research and received advice from each other and from the mentor. A social gathering for all the students was held in the mentor’s home twice during the year. The gathering included dinner and a spiritual message in addition to socialization.
Publications resulting from students mentored during the period of this MEG (indicated in Bold)
Articles
Published (from data gathered during previous MEG):
- Nelson, J., Berbert, A.M., Gibbons, E., Pickett, K.R., Streeter, M., Warcup, A.O., Yeung, C.H.-Y., Judd, A.M., and Bell, J.D. Relationship between Membrane Permeability and Specificity of Human Secretory Phospholipase A2 Isoforms during Cell Death. Biophys. Biochim. Acta 1808, 1913-1920.
In revision after a favorable review:
- Nelson, J., Francom, L.L., Anderson, L., Damm, K., Baker, R., Chen, J., Franklin, S., Hamaker, A., Izidoro, I., Moss, E., Orton, M., Stevens, E., Yeung, C., Judd, A.M., and Bell, J.D. Investigation into the Role of Phosphatidylserine in Modifying the Susceptibility of Human Lymphocytes to Secretory Phospholipase A2 using Cells Deficient in the Expression of Scramblase. Biophys. Biochim. Acta (in revision)
In preparation for submission later this year or early in 2012:
- Nelson, J., Francom, L., Barlow, K., Beck, D.O., Berbert, A., Damm, K., Eschenroder, N., Neeley, K., Pruitt, M., Thompson, K., Thurber, B., Yeung, C.H.-Y., Judd, A.M., and Bell, J.D. Chemotherapeutic apoptosis: who assailed the membrane, the inducer or the induced? (in preparation)
- Gibbons, E., Pickett, K.R., Streeter, M., Warcup, A.O., Nelson, J., Judd, A.M., and Bell, J.D. Biophysical basis for changes to the cell membrane detected by merocyanine and laurdan fluorescence during cell death. (in preparation)
- Franchino, H.B., Stevens, E., Izidoro, I., and Bell, J.D. Relationships between bilayer phase and equilibration rates of patman and laurdan. (in preparation)
- Campbell, L., and Bell, J.D. Membrane properties involved in calcium-stimulated microparticle release from the plasma membranes of S49 lymphoma cells. (in preparation)
- Damm, K., Gibbons, L., Anderson, L., Melchor, S., Nelson, J., Judd, A.M., and Bell, J.D. Role of membrane oxidation in controlling the activity of secretory phospholipase A2 toward apoptotic lymphoma cells. (in preparation)
Abstracts at the Biophysical Society Meeting:
- Gonzalez, L.J., Gibbons, E., Bailey, R.W., Fairbourn, J., Nguyen, T., Smith, S.K., Best, K.B., Nelson, J., Judd, A.M., and Bell, J.D. (2011) The influence of membrane physical properties on microvesicle release in human erythrocytes. Biophys. J. 100 (suppl), (#202).
- Nelson, J., Berbert, A.M., Gibbons, E., Pickett, K.R., Streeter, M., Warcup, A.O., Yeung, C.H.-Y., Judd, A.M., and Bell, J.D. (2011) Biophysical basis for specificity of action of human isoforms of secretory phospholipase A2 during cell death. Biophys. J. 100 (suppl), (#214).
- Franchino, H.B., and Bell, J.D. (2011) Relationships between bilayer phase and equilibration rates of patman and laurdan. Biophys. J. 100 (suppl), (#3410).
Accounting of funds
| Expense Category | Proposed | Actual |
|---|---|---|
| Undergraduate Wages and Benefits | $14,000 | $14,000 |
| Supplies | $4,000 | $4,610.57 |
| Other (flow cytometry and microscopy facility charges) | $2,000 | $1,389.43 |
| Total | $20,000 | $20,000 |