Nathan La Monica and Dr. Dixon Woodbury, Physiology & Developmental Biology
SNAP-25, a protein that is found abundantly in the brain, is a key player in the process of releasing neurotransmitters. Vesicles, or small spheres of lipid membranes, contain neurotransmitters and have numerous proteins that extend outward from the vesicle. To release the neurotransmitters, proteins on the vesicle bind with proteins on the pre-synaptic membrane causing the fusion of the two membranes. SNAP-25 along with Syntaxin (membrane bound protein) and Synaptobrevin (vesicle bound protein) work together to form a complex called the SNARE complex (Bowen, 2004). The current theory on how this complex works is that SNAP-25 has to be bound to the pre-synaptic membrane to participate in the SNARE complex. However, after finding that SNAP-25 naturally exists on the vesicle, Dr. Dixon Woodbury began studying to see if SNAP-25 can also act as a vesicle bound protein. Some of Dr. Woodbury’s preliminary data suggests that SNAP-25 may act in such a manner; an ORCA grant was awarded to continue the study of SNAP-25 acting as a vesicle bound protein.
A brief description of the methodology that I am using is necessary to understand the results. I create a lipid bilayer over a small hole that acts like the pre-synaptic membrane. By running a current through the membrane and adding modified synaptic vesicles (MSV’s), which are rat synaptic vesicles with a substance added, to the surrounding liquid I can tell when the vesicles fuse to the lipid bilayer. When an MSV fuses to the bilayer a voltage spike occurs on the computer measuring the assay.
The first step in the assay is to obtain synaptic vesicles, which we are able to get from extracted rat brains. Through several hours of processing and purifying we extracted several hundred microliters of purified synaptic vesicles suspended in a sucrose solution. Following the extraction of the synaptic vesicles we determined that to create validity for our results we would have three test batches. One batch was treated with BoNT (batch A), which clips SNAP-25 from the vesicle. Another batch was also treated with BoNT, but was treated in the presence of a chelating agent (an agent that binds the zinc which BoNT needs to clip the SNAP-25). The purpose of this batch (batch C) is to see if the BoNT itself has a fusiogenic influence on the proteins. The final batch was mock treated, thus acting like we treated it with BoNT, but just adding a simple saline solution to the batch leaving SNAP-25 intact. After we treated the synaptic vesicles we combined them with artificial vesicles, making modified synaptic vesicles (MSV’s).
Following the treatment of these batches, we ran a Western Blot to determine the amount of SNAP-25 that was clipped with the BoNT (batch A) and the BoNT with chelating agent (batch C), when compared to the mock treated (batch B). The western blot revealed that even with the chelating agent binding the some of the zinc, the BoNT ended up clipping as much SNAP-25 as the batch that included solely BoNT. This occurrence we reasoned to the fact that the BoNT binds the zinc tightly so is not likely to release the zinc in the short time period it was in the presence of the chelating agent. Batches A and C were clipped to contain about 50% of the amount of SNAP-25 present in the mock treated batch B.
Before we run the assay, the protein Syntaxin is added into the bilayer membrane. With Syntaxin in the bilayer membrane all the proteins are present that are needed to make the fore-mentioned SNARE complex, but instead of SNAP-25 also being in the bilayer membrane it is on the mock treated vesicles. The BoNT treated SV’s act as a control. The results of experiments that were run are graphed in Chart 1.
The fusions that occur during the 0.25 gradient are considered spontaneous fusions, meaning the fusions occur through the creation of a SNARE complex. We add a salt gradient to the solution to give a little energy to help the vesicles fuse. The 1.25 gradient adds a significant amount of energy and assists even more vesicles to fuse, so not all of the fusions are SNARE driven. The results of these experiments leave a lot to be desired. We do see a higher fusion rate for the mock treated vesicles (see chart 1 batch B) than for the other two batches. However, the ratio of spontaneous to induced are almost identical for all three batches. The reason the ratio is so important is that no matter how hard we try each experiment will be different. The vesicles will be different sizes, different amounts of Syntaxin will be incorporated in the bilayer, and the hole in the cup will vary in size. So although the mock treated has a significantly higher fusion rate that alone is not proof.
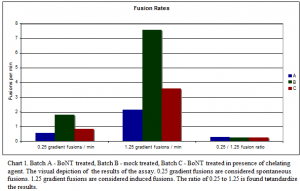
This batch of data is a step closer to proving or disproving our hypothesis that SNAP-25 can act as a v-SNARE. The fusion rate different between mock treated and the other treated vesicles is promising, but more data is needed before we can continue on in confidence. We are currently working on creating more batches of MSV’s with some SV’s Dr. Woodbury obtained from some colleagues in Germany. We hope to test to see if we get the same results with their SV’s to show that our process is indeed appropriate for obtaining SV’s.
We hope to have enough data collected by the end of this summer to begin working on a paper to submit for publication. I have enjoyed the opportunity working with Dr. Woodbury, and will always value the time I had working to bring to light the function of SNAP-25 during career here at Brigham Young University. Thank you for providing me with the financial means to do so.
Sources
- Bowen, M.E., Weninger, K., Brunger, A.T., and Chu,S. “Single Molecule Observation of Liposome-Bilayer Fusion Thermally Induced by Soluable N-Ethyl Maleimide Sensitive-Factor Attachment Protein Receptors (SNAREs).” Biophysical Journal. 87 (2004): 3569-3584.
