Karl A. Kirby and Dr. Kim L. O’Neill, Microbiology
Acetaminophen is a popular non-prescription analgesic and antipyretic drug. Therefore, studies concerning its effects on human DNA are of great value. In this study, the single cell gel, or comet assay, was used to study the extent of Acetaminophen induced DNA damage at varying concentrations and times. The human lymphocyte Raji cell line was used for the experiments.
The comet assay is a procedure that allows the evaluation of single cells under a fluorescent microscope. The alkaline comet assay is used primarily to detect single strand breaks and the neutral assay to detect double strand breaks.1
Initial studies using the alkaline assay indicated that concentrations far exceeding therapeutic doses were necessary to incur any detectable DNA damage. Therapeutic doses result in human plasma levels of 0.07-0.2 mM.3 When damage was finally detectable, the image was more indicative of double strand DNA breaks and apoptosis rather than single strand breaks. In apoptosis, or programmed cell death, cell DNA is cut up by digestive enzymes resulting in massive fragmentation. Consequently, a neutral assay was used to further evaluate acetaminophen induced DNA damage.
At 60 mM acetaminophen exposure, 21.3% of the cells assayed displayed apoptotis-like DNA damage. At the same time, only 4.5% of the same cells displayed Trypan Blue uptake. Trypan Blue is a dye only allowed into cells when they die and is thus used for a viability test. In apoptosis, dye uptake does not immediately follow cell fragmentation, while a cell that dies by necrosis (unprogrammed cell death due to intolerable environmental changes) immediately allows the dye inside. Therefore, the lag time between DNA damage and cell death further supported an apoptotic pathway. This result was not entirely surprising because other non-steroidal anti-inflammatory drugs (NSAIDS) have also been shown to cause apoptosis.2
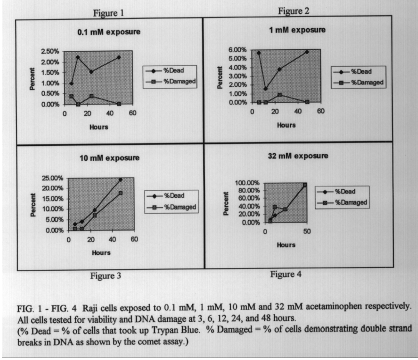
For further evaluation, cells were tested using longer time periods (6, 12, 24, and 48 hrs.) and a range of lower drug concentrations (0. 1, 1, 10 , and 32 mM). In this case, the percent of cells with damaged DNA closely correlated with the percent of cells with dye uptake, or dead cells. This suggests that, under these concentrations and times, cell death may have taken place by necrosis with massive DNA fragmentation rather than apoptosis.
Cells exposed to acetaminophen may undergo cell death by both apoptosis or necrosis depending upon the time of exposure and dosage. Further studies and tests are needed to evaluate the pathways leading to the massive DNA fragmentation detected by the comet assay. Overall, significant DNA damage is only detectable under conditions far exceeding therapeutic doses.
References
- D.W. Fairbairn et. al. (I 995) Mutation Research 339, 3 7-59.
- X. Lu et. al. (I 995) Proc. Natl. Acad. Sci. 92, 7961-7965.
- U. Rannug et. al. (I 995) Mutation Research 327, 179-200.
- Thanks to Bryan Poe and Darcy Fairbairn of Kim L. O’Neill lab for consultation and technical assistance.