Brandon Forbes and Dr. Dixon Woodbury, Department of Physiology and Developmental Biology
Throughout our lives we often take our wonderful nervous system for granted. We simply accept that it will do its job and help us to organize our thoughts into plans for the day, or research reports such as this. At a cellular level, the SNARE proteins mediate fusion of neurotransmitter containing vesicles to the plasma membrane of a neuron to release neurotransmitter. This exocytotic process is part of the orchestra of events that enable us to think. There are three main proteins of the SNARE protein complex with many other proteins playing a role as well. VAMP is associated with the vesicle, syntaxin is associated with the plasma membrane and a third protein whose function or association is not as well understood called SNAP-25. My research project with Dr. Woodbury was focused on SNAP-25 in particular. The neuron naturally contains different percentages of cholesterol by mol ratio throughout the plasma membrane as well as in the neurosecretory vesicles. Dr. Woodbury and I were very interested in the effect of cholesterol on SNARE mediated vesicular fusion.
In our lab, we created artificial vesicles (AV) that did not contain SNARE proteins in order to provide a SNARE free control. These vesicles also contain nystatin/ergosterol complexes that allow us to electrically measure when the vesicle fuses as it causes a spike in electric current crossing the membrane. These same vesicles are then mixed with synaptic vesicles derived from the brain of a rat in order to mix in rat SNARE proteins into the artificial vesicles to create a modified synaptic vesicle (MSV). The target membrane can be manipulated by either including or excluding syntaxin and then varying the mol% of cholesterol contained in the target membrane. Each experiment consists of adding vesicles to a chamber that has a very low salt gradient (150:220 mM KCl) across the phospholipid bilayer. This gradient is not strong enough to induce fusions in a cholesterol free membrane. This allows us to see a “spontaneous” fusion rate in which fusions either happen by pure chance or by SNARE mediated mechanisms. To give us a frame of reference, we then add a high salt gradient across the bilayer (150:473 mM KCl) in order to establish the maximum possible fusion rate, or “induced” fusion rate, under the experimental conditions present. The final product of the experiment is a comparison of the “spontaneous” and “induced” rates to give the “relative” fusion rate. We simply divide the spontaneous rate by the induced rate to give the relative rate. This basic experiment is repeated many times to generate the data that we use to make our conclusions.
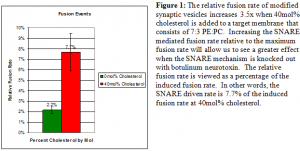
In preliminary experiments, we have seen a very nice increase in SNARE mediated fusion by adding 40mol% cholesterol to the target membrane. In figure 1, we are able to demonstrate a nice 3.5x increase in the relative fusion rate from 0mol% cholesterol to 40mol% cholesterol. This gave us great hope that we have found a way to increase the strength of our relative fusion rate for other projects as well. In later experiments, we noticed that the induced fusion rates of both artificial vesicles and modified synaptic vesicles were higher in cholesterol containing target membranes. Also, the incidence of non-SNARE mediated fusion, or AV fusions, at a low salt gradient increases as well. We have observed that cholesterol will allow artificial vesicles normally considered to be improperly sized for fusion to fuse to the target membrane anyway whereas the same batch of vesicles will not fuse to a control target membrane that lacks cholesterol. Cholesterol therefore increases the likelihood of fusion in general and not just for SNARE mediated fusion. It is important to determine just exactly how much adding cholesterol affects the spontaneous fusion rate. Experiments with no salt gradient may have to be employed if it is determined that a 150:220 mM gradient will reliably generate osmotic vesicle fusions in our assay. This is an ongoing project in the Woodbury lab and experiments are still needed to refine the use of cholesterol in our target membranes.
This project was represented in a research poster at the 53rd Annual Meeting of the Biophysical Society in March of 2009. We are hopeful that the use of cholesterol in the target membrane, which simulates a more physiologically natural membrane, will enable us to increase our power to examine the role of SNAP-25 in SNARE mediated fusion. A future project with this research will be to clip the SNAP-25 on synaptic vesicles with botulinum neurotoxin and then subject these clipped vesicles to a target membrane containing cholesterol and see a more dramatic effect. It is hoped that this research will be part of a larger project to be published in the Biophysical Journal or comparable scientific literature.