Douglas Bennion and Dr. Jeffrey Edwards, Physiology and Developmental Biology
As a new and untried undergraduate researcher, I aspired to do something to impact the lives of others through a modest research contribution of my own. While I have not lost that aspiration, I have been \ surprised to discover that, in fact, others have been the ones making the greater impact in my life. I offer my deepest thanks to Dr. Edwards for his endless patience in explaining time after time things that I should have already known. His impromptu diagrams will always be the first pictures my mind calls up in moments of explanatory need. I also express appreciation to my lab partners, without whose hundreds of hours in the lab, I would not have but one tenth of the data to use in writing the honors thesis that resulted from my research; namely, Jason Couch, Mike Castle, Steve Daniel, Blake Nelson, Matt Mors, and Curtis Walther. I would most especially like to thank Tyron Jensen for being a most reliable partner and friend time and again during the research, presentation, and writing stages of this work. I also thank the Office of Research and Creative Activities for placing their trust with me in the form of two ORCA grants. Finally, my deepest love and gratitude I give to my wife, Jenn. I cannot recall a single word of complaint on her part against the long hours and late nights that I devoted to the project. The research has been published in Chiasm, BYU’s Undergraduate Journal of Neuroscience, as an honors thesis, and has been presented as an award winning research poster at several research conferences, including two international Neuroscience conferences.
Central to all human knowledge, creative thought, and life experience is the brain, the most incredible processing unit known to mankind. Brain research, ranging from the evaluation of complex emotional disorders to detailed descriptions of neuronal pathology, is considered by many to be the last and greatest frontier in health research. The nervous system receives and processes external stimuli in a time so short it is only just measurable. Following this lightning-quick decision making process, the cortex acts through the peripheral branch of the nervous system to send commands to distal parts of the body. The electrical signals race down a complex pathway of nerves and cell bodies to where they innervate various tissues and elicit concerted responses. These responses can include the participation of sometimes hundreds of separate members, such as a quick series of muscle movements to steer clear of danger or a sharp intake of breath and a racing pulse in a moment of surprise. This processing and communication often occurs in less time than the blink of an eye. Perhaps even more remarkable than this processing power, however, is the brain’s ability to store literally billions of pieces of information, both short-term and long-term, in the form of vivid memories within a single human mind.
Learning and memory are phenomena made possible via physical changes at neuronal synapses in the brain, a process known as synaptic plasticity. Because synaptic plasticity affects physical changes in the brain in response to external stimuli by strengthening or weakening specific neural pathways, it is suspected of playing an essential role in regulating short-term learning and memory. Dysfunctions in synaptic plasticity contribute to such diseases and disorders as Alzheimer’s Disease, Parkinson’s Disease, and retrograde amnesia. By further clarifying the mechanism of two specific forms of synaptic plasticity, long-term potentiation (LTP) and long-term depression (LTD), in which the transient receptor potential cation channel, subfamily V, member 1 (TRPV1), or the “hot-pepper receptor,” is suspected of participating, I hope to fill part of a research gap that may bring science and medicine a step closer to producing effective treatments for such debilitating illnesses.
TRPV1 is a ligand-gated cation channel located throughout the central nervous system whose mechanism of action is not clearly understood. Other subtypes of TRP channels are located in the peripheral nervous system (PNS), and these receptors are heavily researched due to their role in inflammation and the transmission and modulation of pain. TRPV1 channels are activated by the binding of capsaicin, a crystalline compound that is also responsible for producing the heat of red peppers. Hence, this receptor has earned the nickname of being the “hot-pepper receptor.” TRPV1 is activated in a variety of other ways, including activation by heat in excess of 43 °C, and low pH (acidic conditions). Certain other receptors that mediate synaptic plasticity have been extensively studied, specifically the N-methyl-D-aspartate (NMDA) and metabotropic glutamate receptors, while the TRPV1 receptor, which appears to also modulate plasticity, has received far less attention.
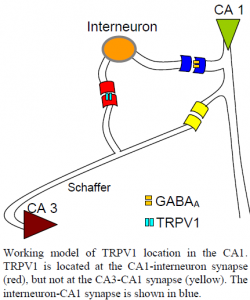
The research in which I participated resulted in experimental findings regarding the role of transient receptor potential cation channel, vanilloid 1 (TRPV1) in modulating the mechanism underlying learning and memory in the hippocampus. Much of LTP research has been conducted in the hippocampus, a declarative memory and learning center, where TRPV1 receptors have recently been shown to modulate synaptic plasticity. Using field recordings obtained from rat brain slices, we measured synaptic currents from hippocampal CA1 pyramidal cells to examine the effects of TRPV1 activation in enhancing LTP. Our findings indicate that TRPV1 activation via TRPV1 agonist capsaicin enhances LTP among pyramidal cells of the CA1. We report that blockade of inhibitory interneurons by application of GABAA antagonist picrotoxin results in a blockade of TRPV1s enhancement of pyramidal cell LTP in the CA1. We propose that the observed LTP enhancement in the presence of capsaicin occurs through inhibition of GABAergic CA1 interneurons by TRPV1 activation at the CA3-interneuron synapse. These findings shed additional light on the largely unknown mechanism driving spatial and declarative memory and learning.