Christopher M. Runyan and Dr. Ronald Leavitt, Microbiology
Introduction
Colibacillosis is a disease found in poultry that results in various levels of morbidity and mortality. It is primarily caused by pathogenic strains of E. coli that inhabit the poults’ intestinal tracts. Antibiotics have frequently been used to protect the turkeys but with less and less effectiveness as the bacteria have begun to develop antibiotic resistance. Hence alternate ways are needed to combat pathogenic bacteria.
Bacterial interfering agents are organisms that can outcompete potential pathogens. For example, some strains of Escherichia coli are more likely to colonize a turkey’s intestines than others although their introduction to the gut may occur simultaneously. Additionally, some of these interfering agents produce proteins known as colicins or bacteriocins that also prevent the growth of other strains. Our lab has found that strain 38 of E. coli in turkeys produces a small but powerful bacteriocin. This microcin inhibited 58 of 59 strains of E. coli and 30 of 32 strains of Salmonella. However, strain 38 is ineffective in permanently replacing pathogenic strains as the normal flora because it adheres poorly to the intestinal walls.
Other research in our lab found that the ability of E. coli to adhere to the intestinal walls is attributed to a plasmid-encoded protein. A former undergraduate demonstrated this by curing high-adhering strain 28 of a 106 kilobase-pair (kbp) plasmid and by showing a subsequent loss of the bacteria’s adherence capability. A sounder understanding of the high adherence plasmid, pHA28 would enable us to construct a microcin and adherence-positive strain of E. coli that would greatly inhibit pathogens in turkeys. This would then potentially eradicate colibacillosis in turkeys and could then potentially be applied to chickens. My project was to find the gene that codes for the high adherence proteins, and ultimately to transfect the gene into the microcin producing strain 28, and to test the interfering agents on turkeys.
Procedures
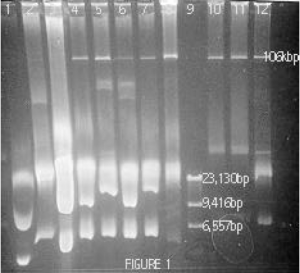
The first step in this project was to refine a plasmid preparation procedure that gave good yields of this strain’s high adherence plasmid (pHA28). The most common procedures involve an alkaline-lysis step in which bacterial cells are lysed open with lysozyme and sodium hydroxide. The sodium hydroxide then breaks the forces between the two strands of DNA, causing all chromosomal and plasmid DNA in the cell to denature or “unzip”. Potassium acetate is then added to restore the original pH level, allowing plasmid DNA to “zip” back together while leaving the complex chromosomal DNA in a jumbled glob that can be removed with the detergent SDS as well as through phenol extractions. In the protocol used by former students the preparations were incubated on ice for 5 minutes during exposure to potassium acetate. By changing variables one at a time, I found that an incubation period of 30 minutes at 55 C during this step gave the large adherence plasmid more time to reanneal, resulting in higher yields (compare lane 1 with lanes 4-8 in Figure 1).
With this plasmid preparation protocol I was able to consistently remove all of the DNA from strain 28. The next step was to isolate pHA28 from the remaining chromosomal DNA (at ~23kbp in figure 1) as well as from the 2 small plasmids carried in strain 38 (one at ~5kbp and the other not shown). I tried 4 different methods of isolating pHA28. All of these involved running a preparative gel to separate the DNA as shown in figure 1. The genetic material from the plasmid preparation was stained with ethidium bromide for viewing under UV light and the strip of agarose containing pHA28 was visually identified by comparison to a genetic ladder (lane 9, Figure 1) and was then excised from the gel. The agarose was then either 1) melted in a hot water bath to release pHA28 to allow its retrieval by salt precipitations after removing agarose proteins with phenol extractions, 2) digested using the enzyme, â-agarase, allowing pHA28 to be precipitated using ammonium acetate, 3) dissolved by sodium iodine allowing pHA28 to be precipitated using sodium acetate, or 4) exposed to additional electrophoretic current in order to run pHA28 out of the gel into a membrane-enclosed buffer so that it could be retrieved through a salt precipitation. (DNA can be retrieved from a large volume of liquid by treating it with a compound with which it can form a salt and by adding ethanol to allow the aqueous salt to precipitate.)
These plasmid isolation methods, though proven elsewhere, were not successful in helping me retrieve pHA28. Running electrophoretic gels of the samples collected from these protocols showed that none of the intact 106-kbp plasmid was isolated, and faint bands of DNA at about 31kbp suggested that the plasmid may be being knicked, in which a single phosphodiester bond is broken. Ethidium bromide-bound DNA is highly susceptible to knicking when exposed to light. When supercoiled plasmid DNA is knicked it unwinds and may denature. To minimize this I stained only the first few lanes of each preparative gel, which were then used to estimate the location of the unstained pHA28 DNA in adjacent lanes. The pHA28 not exposed to ethidium bromide was then used in the 4 isolation protocols mentioned previously, but still no plasmid was retrieved.
My research advisor and I hypothesize that pHA28 has abnormal characteristics that may make it highly susceptible to shearing and knicking using our plasmid preparation protocols. My next course of action will be to investigate methods of isolating plasmids that do not involve alkaline cell lysis and the use of a detergent to separate double-stranded DNA. Gentler methods such as sucrose-lysozyme gradient protocols may prevent the mechanical shearing and chemical knicking of pHA28 that seems to have hindered our progress.
