Jason T. Rich and Dr. James D. Porter, Zoology
The purpose of my study was to quantify the effects of angiotensin II on young rats under extreme stress. We hypothesized that we would observe an angiotensin II-mediated switch in brain hormonal expression from corticotropin-releasing hormone (CRH) to arginine vasopressin (AVP). Ten rats were equipped with osmotic ventricular pumps. Five rats received losartan (an angiotensin II antagonist) and the other five received a saline solution. Three rats where left untreated, to act as a control for the experiment. Two losartan treated rats and one saline treated rat died during the stressing period. However the losartan pumps were salvaged and implanted into two of the control rats. After the two-week stressing period, the pumps were checked for proper perfusion and the rats were sacrificed. In the end we were left with four losartan treated rats, three saline treated rats, and one control rat whose tissue was viable for RT-PCR testing.
Tissue samples from the hypothalamus were taken and put in deep freeze. mRNA was subsequently extracted from the samples and converted to DNA through RT reactions. We ran PCR reactions for 18S mRNA and angiotensin II receptor (AT1) mRNA in order to test for the presence of mRNA. These experiments proved without a doubt that the samples contained mRNA and that the mRNA was in good condition (see Figure 1).
The quantification of CRH and AVP mRNA proved to be the most difficult step in this study. Our challenge was to create effective primer pairs for CRH and AVP, since no primer pairs were commercially available for these two hormones. We first attempted to create our own primer pairs for CRH based on the known mouse CRH gene sequence; unfortunately these primer pairs only worked sporadically. We then researched scientific journals and found published primer pairs for both hormones.1 However, we could not obtain the same published results, even after many months of arduous testing and retesting.
The AVP primer pairs amplified the appropriate sequence, yet proved to be sporadic and unpredictable in their success rate. Since we were able to get the primer pairs to work in a few experiments, we concluded that the problem stemmed from incorrect PCR steps and reagents rather than from faulty primer pairs. At present we do not have any AVP data for our tissue samples, but with further manipulation of the PCR reaction we feel confident that we will soon be able produce accurate and predictably results.
The CRH primer pairs produced strong bands of amplified DNA. Unfortunately the amplified sequence did not include the CRH gene. The CRH primers were documented to amplify a 365 bp fragment, but our amplified fragment turned out to be 472 bp in length (see Figure 1). DNA sequencing showed multiple partial-homologies with the rat genome, but none of the homologous segments included the CRH gene. It appears that we have found a new undocumented DNA sequence in the rat genome. The next step will be to find new CRH primer pairs and retest the samples.2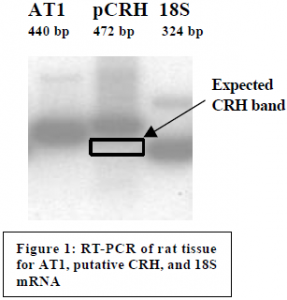
References
- Wang, H.L., Tsai, L.Y., and Lee, E.H.Y. (2000) Corticotropin-releasing factor produces a protein synthesis-dependent long-lasting potentiation in dentate gyrus neurons. JNeurophysiol 83(1): 343-9.
- LeMoullec, J.M., Jouquey, S., Corvol, P., and Pinet, F. (1997) A sensitive reverse transcriptase polymerase chain reaction assay for measuring the effects of dehydration and gestation on rat amounts of vasopressin and ocytocin mRNAs. Molecular and Cellular Endocrinology 128, 151-9.
Acknowledgements
I would like to thank Dr. Porter for his patient teaching and guidance, the which made this whole project possible. Also, I thank the many lab assistants in Dr. Porter’s lab for the long hours they put in to helping me complete this project.
