Jason Meyer and Dr. Gregory Burton, Microbiology
The use of highly active antiretroviral therapy (HAART) for HIV-infected patients can result in the reduction of plasma viral RNA levels to nearly undetectable levels. However, the existence of replication competent virus from chronically infected CD4+ T cells despite apparently successful HAART treatment suggests the existence of protective reservoirs in the body that protect virally infected cells from the effects of HAART treatment (1). Follicular dendritic cells (FDC) are specialized cells that exist primarily in secondary lymphoid tissue. The purpose of my research was to examine my hypothesis that FDC act as such reservoirs by protecting infected cells from the effects of HAART treatment.
In order to test my hypothesis, drugs that are routinely used in HAART were prepared for use in my in vitro culture system. AZT, a nucleoside analog, is effective at inhibiting the reverse transcription step of the HIV life cycle. Crixivan is an agent that inhibits the activity of the HIV protease, which cleaves a long viral polypeptide into various structural components of the virion. According to previous studies, concentrations of 0.2 mM AZT have been shown to be sufficient to inhibit viral production while concentrations up to 0.16 mM Crixivan have been shown to be well-tolerated by PBLs (2, 3). Several dilutions of the drugs were made using these considerations.
Once the HAART drugs, AZT and Crixivan, were prepared, peripheral blood lymphocytes (PBL) were isolated from a healthy volunteer using the ficoll-hypaque density gradient centrifugation technique. 1´105 PBLs were aliquoted into several culture tubes containing AZT, crixivan, or a combination of the two drugs.
The cultures were next separated into three different groups. The first group served as a control as no FDC’s or FDC depleted cells were added. The second group of these cultures was incubated with 1´104 FDC, which were isolated from the tonsils of an otherwise healthy donor. The last group was incubated with 1´104 FDC-depleted cells. After 12 hours of incubation, 50 mL of HIV-IIIB was added to the cultures.
In order to determine the effects of FDC on the actions of AZT, cultures were incubated for 5 and 14 days. Integration of the viral genome into the PBLs was measured using polymerase chain reaction (PCR) using probes specific for the HIV sequence, gag. The amplified DNA fragments were then resolved on an agarose gel through electrophoresis and a Southern blot was performed. No bands were visible for the HIV infected PBLs without AZT. However, in the FDC co-cultures, faint bands were observed (Figure 1).
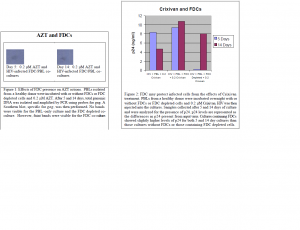
To examine the effects of FDC on Crixivan actions, supernatants of the cultures were isolated after 5 and 14 days of incubation. A p24 ELISA was then performed to determine the concentration of this HIV protein. Again, there was not a significant increase in virus production in the control culture, which contained only PBLs and HIV. The FDC co-cultures and FDCdepleted co-cultures, however, showed considerable increase in viral production (Figure 2).
Unfortunately, the results of my research do not really provide any conclusive evidence that FDC protect or don’t protect infected cells from the effects of HAART therapy. Most discouraging was the fact that my control groups did not show active infection. The best way in which to correct this problem is to have many more duplicate control cultures so that errors associated with DNA isolation or p24 analysis are eliminated as possible causes. In addition, p24 data was not consistent, possibly reflecting pipetting error associated with the setup of the cultures. This error may be minimized by utilizing methods in which to set up the cultures with as few pipetting steps as possible.
References
- Furtado, M. R. Callaway, D. S., Phair, J.P., Kuntsman, K. J., Stanton, J. L., Macken, C. A., Perelson, A.S., and S.M. Wolinsky. 1999. Persistence of HIV-1 transcription in peripheral-blood mononuclear cells in patients receiving potent antiretroviral therapy. N. Engl. J. Med. 340: 1614-1622.
- Merril, D. P, Manion, D. J., Chou, T., and M. S. Hirsch. 1997. Antagonism between human immunodeficiency virus type 1 protease inhibitors indinavir and saquinavir in vitro. J. Infect. Dis. 176: 265-8.
- Merril, D. P., Moonis, M., Chou, T., and M. S. Hirsch. 1996. Lamivudine or stavudine in two- and three-drug combinations against human immunodeficiency virus type 1 replication in vitro. J. Infect. Dis. 173: 355-64.