Jason D. Merrell and Professor David D. Busath, Zoology
After discussion with my faculty mentor, together we decided that my original ORCA proposal would not best suit the needs of the laboratory in which I am allowed to work. Upon reaching this conclusion, a new research plan was organized. The following outlines the progress of this new plan and demonstrates the on-going research that was facilitated via my ORCA scholarship.
Grotthus proton conductance is observed to occur in membrane proteins1. This method of ion displacement across phospholipid bilayers is of interest to the scientific community, for the study of such will aid in understanding actual naturally occurring phenomena in living cells. Gramicidin M (gM), a membrane protein that is an analog of the more commonly-known gramicidin A (gA), differs from the latter due only to replacement of all four Trp residues at positions 9, 11, 13, and 15 with four Phe residues at the same positions. This replacement is significant because Trp possesses a dipole moment; Phe is usually considered to be non-polar. In a recent publication it was noted that “replacement of one or more Trps with Phe enhances proton transport2″. This publication attempts to draw the above conclusion based on experiments measuring conductance in phospholipid bilayers made of two differing molecules: glycerylmonoolein (GMO) and diphytanoylphosphatidylcholine (DPhPC). This conclusion can indeed be reached in the case of proton conductance through gM in GMO, for it was shown by Phillips et al. that proton conductance through gM in GMO is higher than proton conductance through gA or through any other gA analog in GMO in which one or more of the Trp residues has been replaced with Phe surrounded by 0.1 N HCl with an applied potential of 50 mV.
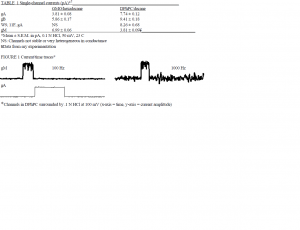
However, in my research, I found that there is an exception to the broad conclusion made by Phillips et al.: in DPhPC bilayers surrounded by a 0.1 N HCl solution, proton conductance through gM with an applied potential of 50 mV is significantly lower than proton conductance through gA or any other gA analog mentioned (Table 1). Furthermore, when such gM channels in DPhPC bilayers demonstrating Grotthus conductance are open, they display a highly increased amount of noise that is disproportionate with the amount of noise when the same channels are closed. This increased amount of open-channel noise does not occur in GMO with any gA analog surrounded by KCl, NaCl, or HCl solution, nor in DPhPC with gA surrounded by 0.1 N HCl. This must mean that some type of interaction between protons, DPhPC, and gM causes a distortion of the gM channel that produces this channel phenotype with characteristic high openchannel noise (Fig. 1). Channels demonstrating this phenomenon have not been previously reported. Perhaps the high amount of noise is a reason why there exists a discrepancy in the conclusion reached by Phillips et al.
In order to show that proton conductance through gM channels in DPhPC has been fully discerned by the lab’s equipment, that the reason why a channel displaying higher conductance does not appear on a current vs. time trace is not due to the inability of the equipment to discern a very rapidly alternating current when the channel is open (i.e. channel noise), the filter that is set between the electrodes in the 0.1 N solution and the computer collecting data was used as a positive control: experiments were performed at 100 mV with the filter’s corner frequency set at 100 Hz and then at 1000 Hz (Fig. 1). There did not appear a recorded significant increase in conductance, indicating that the lab’s equipment can indeed discern the full conductance of this channel phenotype.
References
- Moore, W.J. 1972. Physical Chemistry. Prentice-Hall, Englewood Cliffs, NJ.
- Phillips, L. R., C. D. Cole, R. J. Hendershot, M. Cotton, T. A. Cross, and D. D. Busath. 1999. Noncontact Dipole Effects on Channel Permeation. III. Anomalous Proton Conductance Effects in Gramicidin. Biophysical J. 77:2492-2501.