Jeffrey C. Markham and Professor David D. Busath, Zoology
The oligopeptide gramicidin is one of the simplest proteins which can form channels in cell membranes to conduct ions into or out of a cell. The peptide is produced by the bacteria Bacillus brevis. It can also be synthesized by scientists for research purposes. By producing slightly modified variants of the standard gramicidin structure, conclusions can be drawn about structurefunction relationships based on experimentation with the variants.
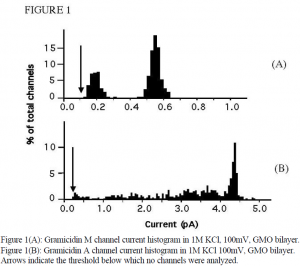
For this project, two closely related gramicidin variants were studied in single channel conductance experiments. Gramicidin A has four tryptophan residues which are located next to the water-lipid interface when in the lipid bilayer. These polar side chains are thought to induce a broad range of reduced conductance channel states (called “mini” channels) in addition to the standard conductance state. To investigate the hypothesis that these “mini” channels are actually induced by the tryptophan side chains, experiments were performed using a gramicidin variant called gramicidin M, in which all of the tryptophan residues are replaced by non-polar phenylalanine residues.
Experiments with both peptides showed that indeed a large portion of the “minis” were absent in gramicidin M. However, a range of “minis” were observed in gramicidin M, with a conductance 30-40% of the standard conductance (see Figure 1). The cause of this range of “minis” is not known, but must be something other than the tryptophan side chains.