Josie Johnson and Dr. Merril J. Christensen, Food Science and Nutrition
Prostate cancer is the most common form of cancer and the leading cause of cancer death in American men.1 Results from a cancer prevention trial performed by Clark et.al.2 show that selenium (Se) supplementation in male subjects reduced cancer incidence by 75%. The molecular basis for the chemopreventive effect of Se merits attention in molecular biology research. For the past six months, my research has focused on the impact of Se-supplemented medium on gene expression of transformed human prostate epithelial cells (entitled LNCaP cells) grown in culture. Through differential display methods, tumor suppressor genes or oncogenes that are either upregulated by high Se levels or downregulated by control Se levels may be determined. Preliminary to differential display, I had to determine the levels of Se supplementation in the form of sodium selenite (Na2SeO3) in cell culture medium that are ideal for control and high Se treatment groups. Three important issues were considered: (1) prostate tissue in the whole organism is only exposed to Se in the blood, so the concentration of Se in the high Se treatment group must be serum-achievable; (2) control Se levels must be high enough to maximize cytosolic selenoenzyme activity; (3) Se supplementation at any level must not be cytotoxic.
Serum Se levels of Clark trial subjects were considered in determining relevant control and high Se supplementation levels for cultured cells. Clark subjects in the control-Se group had average serum Se levels of 114 ng/mL, while the subjects in the high-Se group had average serum Se levels of 190 ng Se/mL. For high Se exposure in cultured cells, it was of interest to exceed the Clark trial high Se level of 190 ng/mL to maximize the probability of differential gene expression. However, as previously mentioned, relevance of the cell-culture model to the whole organism demands that Se supplementation levels be serum-achievable. I conducted a literature search to find results of in vivo studies determining serum-achievable Se levels without symptoms of selenosis. The following are results of two relevant studies: (1) in a seleniferous area of South Dakota, serum Se levels as high as 813 ng/mL were observed without evidence of selenosis;3 (2) in a seleniferous area of China, selenosis symptoms were present in individuals with serum Se levels of 1346 ng/mL, but those symptoms were not present at levels as high as 968 ng Se/mL serum.4 Based on this data, it was decided to expose high Se treatment cells to 600 ng Se/mL in the culture medium. This was considered to be a conservative serumachievable level without inducing selenosis.
For control Se supplementation levels, it was originally anticipated that culture medium supplemented with 100 ng Se/mL, as an approximation of the Clark trial control Se subject serum Se levels of 114 ng/mL, would be ideal. Therefore, the following culture medium supplementation levels were employed for the first experiment: 100 ng Se/mL for control and 600 ng Se/mL for high. Four flasks of cells at each level were exposed for 48 hours. RNA was isolated using Trizol, and then electrophoresed on a 2% agarose formaldehyde gel. On the day of harvest, the control Se group looked normal, but the high Se cells exhibited atypical morphology. Normally LNCaP cells adhere to the culture flask, but the cells in the high Se group were mostly floating. The agarose gel confirmed poor RNA integrity in these cells. Control Se RNA showed the two normal 18S and 28S bands. High Se RNA, however, had a compromised 18S band and almost no 28S band. This preliminary experiment brought attention to the issue of cytotoxicity.
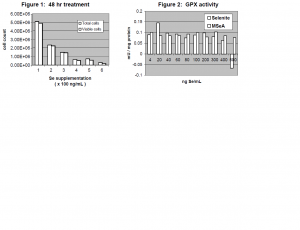
To determine the cutoff for cytotoxicity with Na2SeO3 supplementation, a titration was performed with Se supplementation levels of 100, 200, 300, 400, 500, and 600 ng Se/mL. Trypan blue exclusion assays determined cell viability (see Figure 1). According to these assays, there was a 54% decrease in cell count and viability between 100 and 200 ng Se/mL. It was then decided to perform a second titration with Se levels of 4, 20, 40, 60, 80, 100, 200, 300, 400, 500, and 600 ng Se/mL in two Se forms: Na2SeO3 and methyl seleninic acid (MSeA). Trypan blue exclusion assays on this titration revealed that the most marked decrease in cell count and viability for Na2SeO3-supplemented cells is indeed between 100 and 200 ng Se/mL. Therefore, the high Se supplementation level was changed to 100 ng/mL and the control Sesupplementation level was changed to 4 ng/mL.
In order to ensure that 4 ng Se/mL was sufficient to maximize the activity of cytosolic selenoenzymes, an assay of glutathione peroxidase (GPX) activity was performed, according to the Lawrence and Burk protocol5 (see Figure 2). (GPX was assayed because it is the last selenoenzyme to be supplied with Se in the Se metabolic pathway.) The results of the assay confirmed that GPX activity in LNCaP cells was in fact maximized at Se supplementation levels as low as 4 ng Se/mL.
 Future tasks include exposing four flasks of cells at each Se supplementation level for 24 hours, isolating RNA, performing differential display, isolating differentially expressed genes, sequencing genes to determine identity, and confirming gene identity with reverse transcription polymerase chain reaction (RT-PCR). This will be done for control and high Se levels in the forms of both Na2SeO3 and MSeA. MSeA merits attention as an alternative to Na2SeO3 in Se supplementation for two reasons. First, in vitro studies have found that MSeA induces less cell membrane leakage and facilitates more cell attachment to the flask than does Na2SeO3. Second, MSeA is much closer to methyl selenol (the active chemopreventive Se agent in the cell) in the Se metabolic pathway than is Na2SeO3. It will be of interest to compare these two Se supplementation forms side-by-side in the next experiment.
Future tasks include exposing four flasks of cells at each Se supplementation level for 24 hours, isolating RNA, performing differential display, isolating differentially expressed genes, sequencing genes to determine identity, and confirming gene identity with reverse transcription polymerase chain reaction (RT-PCR). This will be done for control and high Se levels in the forms of both Na2SeO3 and MSeA. MSeA merits attention as an alternative to Na2SeO3 in Se supplementation for two reasons. First, in vitro studies have found that MSeA induces less cell membrane leakage and facilitates more cell attachment to the flask than does Na2SeO3. Second, MSeA is much closer to methyl selenol (the active chemopreventive Se agent in the cell) in the Se metabolic pathway than is Na2SeO3. It will be of interest to compare these two Se supplementation forms side-by-side in the next experiment.
References
- Parker, S.L., Tong, T., Bolden, S., Wingo, P.A.. 1990. Cancer statistics. CA Cancer J Clin. 46:5-7
- Clark, L.C., Dalkin, B., Krongrad, A., Combs Jr., G.F., Turnbull, B.W., Slate, E.H., Witherington, R., Herlong, J.H., Janosko, E., Carpenter, D., Borosso, C., Falk, S., Rounder, J.. 1998. Decreased incidence of prostate cancer with selenium supplementation: results of an ongoing double-blind cancer prevection trial. Brit. Urol. 81: 730-734
- Howe, M.. 1979. Selenium in the blood of South Dakotans. Archieves of Environmental Health. 34:444-448.
- Yang, G., Zhou, R.. 1994. Further observations on the human maximum safe dietary selenium intake in a seleniferous area of China. J Trace Elem Electrolytes Heath Dis. 8:159-65.
- Lawrence, R. A., Burk, R.F.. 1976. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem. and Biophys. Res.Common. 71:952-958.
