David Allan Edwards and Professor R. Paul Evans, Zoology
Within the membrane of excitable cells like those of nerve and muscle tissue, lie proteins called ion channels, which control the passage of ions in and out of the cell. The flow of ions stimulates the action of the cell, which ultimately leads to contraction of muscle tissue in the case of muscle cells, and sensation in the case of nerve cells. Seizures and pain sensation are extreme results of the action of these cells. To control pain, for example, we may use anesthetics that directly interact with ion channels and prevent the flow of ions across the cell membrane. And, in order to design effective drugs, knowledge of the ion channel’s geometry, action, and interaction with other molecules is crucial.
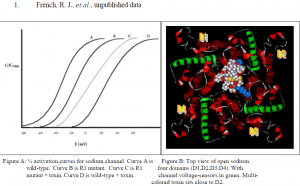
I have studied the voltage-gated sodium channel and its interaction with the toxin protein, u- Conotoxin, from the Pacific snail Conus geographus. u-Conotoxin is one of many toxin proteins being studied for their use in alleviating neuropathic pain. It is a highly charged and very stable protein and interacts with the channel by plugging the hole where ions pass through, effectively preventing cell stimulation. (Fig. B) A useful derivative of u-Conotoxin, R13Q (the same toxin where a positively charged amino acid, arginine 13, is neutralized to glutamate), binds to the Na+ channel but allows for 30 % passage of ions.1 This is useful because it allows for the measurement of other parameters within experiments that either increase or decrease the strength of the channel/toxin interaction (detectable by measuring either an increase or decrease in ion flow).
u-Conotoxin’s many charged amino acids likely interact with the channel’s gating mechanism, which relies on sensing voltage. I decided to look at which voltage-sensing amino acids in the Na+ channel are most affected by the toxin’s presence. First, I applied a voltage to a cell and measured the current passing through normal ion channels. I plotted this as a ½ activation curve (a graph that shows the voltage needed to activate, or open, ½ of the channels) to serve as a control. (Fig. A, curve A) Second, I exposed the cell to R13Q u-Conotoxin and plotted the ½ activation curve. (Fig. A, curve D) Third, I mutated the Na+ channel by neutralizing one of many positively charged amino acids (R1) it uses to detect voltage and activate the channel. This is done through a molecular biology technique called site-directed mutagenesis. I chose an amino acid likely to be the closest to the toxin when it binds, in order to maximize being able to detect any interaction with u-Conotoxin. After mutating the channel and expressing it in cells, I again measured the voltage needed to activate ½ of the channels. The ½ activation curve shifted to the right because it required more voltage to activate the channels in the absence of the amino acid I mutated. (Fig. A, curve B) Lastly, I exposed cells with the mutant channel to u-Conotoxin and measured activation again.
If the increase in required voltage for activation of the normal channel bound to toxin was due to the interaction between the toxin and R1, then a neutralizing mutation of R1 should result in superposition of the resultant ½ activation curve and that of the control. What I saw was a ½ activation curve that fell between the control curves A and B, and the normal channel/toxin curve. Two conclusions can be made from this: one, the toxin does in fact have an effect on the voltage sensing amino acids of the Na+ channel, and two, R1 is not the only amino acid the toxin interacts with to affect gating.
The structure of the toxin protein is well understood as well as its orientation when it docks into the Na+ channel vestibule.1 (Fig. B) Thus, as a key must resemble a keyhole, the shape of the toxin is a negative representation of the shape of the channel pore. The remaining structural geometry of the channel is unknown.
In order to illuminate what the channel looks like outside the pore region I have carried out the above experiment. Here we mutated the most likely amino acid to interact with the toxin probe, an amino acid in domain 2 that appears to lie close in proximity to the toxin when bound. (Fig. B) The future of the project is to create neutralizing mutations of other possible interacting candidates, collect the electrophysiological data, and compare them. Distances between the toxin probe and the amino acids it interacts with can be estimated from the results. A geometric picture of the channel can then be drafted, rather than the theoretical sketch we use now. The more we understand what the channel looks like, and how it acts, the better we will be able to explain and correct the diseases its malfunction results in, whether at a primary genetic level or pharmacologically.