Gongze Zhao, Dr. Laura Bridgewater, Micro & Molecular Biology
Introduction
The human gut consists of approximately 1.5 kg of bacteria, and 50% of the biomass in our fecal matter is bacterial cells (Nicholson, 2005). Diet is a major factor in shaping the composition of the gut microbiota, (Zhang, 2010) which in turn influences the body by producing metabolites that enter the circulation through different pathways. In 2013, by using Koch’s postulates, scientists were able to demonstrate that the gram-negative opportunistic pathogen E. cloacae B29 can cause obesity and chronic inflammation in its host (Fei & Zhao, 2013). Bacteriophage (phage) are viruses that infect bacteria. Phages bind to their bacterial host cells at specific and unique binding sites on the cell surface. They inject their DNA into the bacteria, causing the bacterial cell to fill with new progeny phages that lyse and kill the cell. We hypothesize that the host-specific characteristic of the phages can be utilized to develop a therapy, which can manipulate the gut microbiome by selectively reducing the number of obesity causing pathogen(s) to alleviate metabolic syndrome without damaging the population of probiotic and commensal bacteria.
Methodology
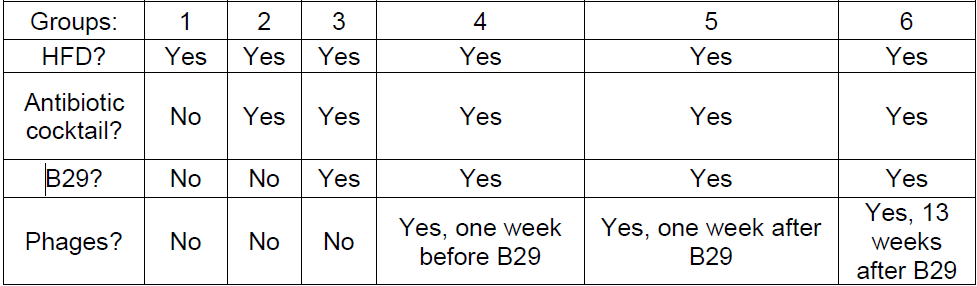
We have already isolated nine different phages that lyse B29, have narrow host range, and are robust enough to transit through the mammalian gut before the start of this project. We attempted to generate obese mice due to the growth of B29 in the gut and high-fat diet (HFD) by first treating mice daily with a concoction of antibiotics (ampicillin, neomycin, vancomycin, and metronidazole) and an antifungal drug (amphotericin-B) for three weeks via oral gavage. Then the mice received rifampicin (to which B29 is resistant) in their drinking water for the duration of the study. B29 was administered to the mice via oral gavage daily for one week, and mice were placed on a high fat diet. Fecal pellets were collected, cultured, and analyzed to verify B29 colonization. Booster gavages of B29 were administered when its levels fell too low. The phage cocktail was then administered to the test group mice via oral gavage. The study will include six groups with eight mice in each group. The groups are the following:
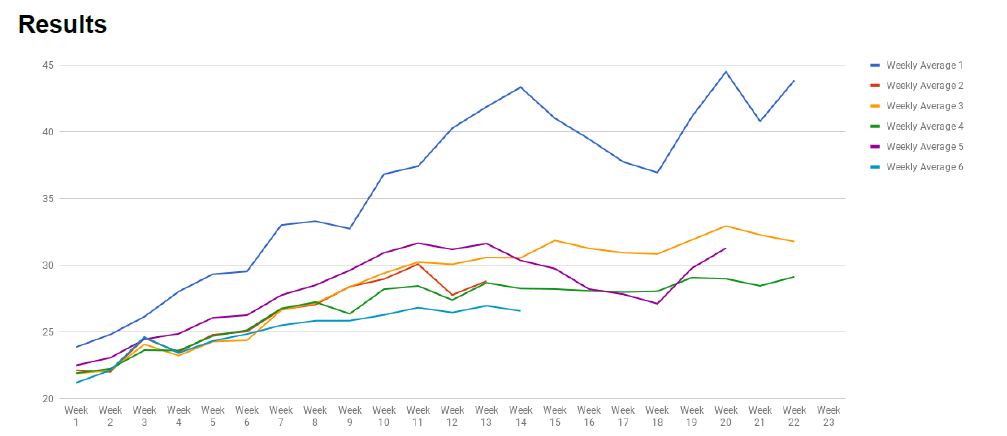
Figure 1 Average weight of each group of mice.
Figure 1 shows that mice that are in group 1 (positive control) were significantly more obese than the other groups. However, the weight differences from the rest of the groups were not significant enough to draw any conclusive results. We have also conducted bi-weekly oral glucose tolerance testing (OGTT) on all mice. The results from the OGTT was also similar to the average weight results, where group 1 was significantly higher than the other groups, but there were no significant differences among the other groups.
Discussion
During the course of the study, we have found that having rifampicin in the mice’s drinking water, even though it is a crucial step in helping to suppress the mice native gut-microbiome, also contributes to making the mice sick from unwanted B29 infection. Because B29 is present in the mice’s fecal pellets, and mice are found to practice coprophagia, B29 is then transferred from the mouse’s gut to the mouth than to the mice’s exposed skin areas such as eyes, ears, and skin through scratching, and even through the rifampicin water. This caused fever and another discomfort in the mice, making them unable to gain weight from B29.
Obese mice models made from B29 colonization exists in germ-free mice, and the effect of obesity is much easier to achieve. However the high cost of maintaining germ-free animals made it impossible for us to use this method. We originally thought that we could create a substitute animal model through using pseudo-germ free mice created from antibiotics to test the effectiveness of our phages, but this study has shown that this animal model doesn’t function as well as we wanted. Therefore, for us to truly test the efficacy of our phage therapy, we would need to perform this test with germ-free animals.