Jacob Anderson, Dr. Chad Hancock; Nutrition, Dietetics, and Food Science
Introduction
Recent studies indicate a correlation between iron dysregulation and insulin sensitivity, suggesting that iron dysregulation may play a role in the development of type 2 diabetes mellitus [1]. However, the connection between iron regulation and insulin sensitivity is not fully understood. Iron is normally tightly regulated, and most iron within cells is bound to prevent aberrant production of free radicals. Non-bound iron is highly reactive via Fenton Reaction chemistry to produce hydroxyl radicals, contributing to oxidative stress and possibly to the development of insulin resistance. Not only does
mismanagement of iron contribute to oxidative stress, but evidence suggests that oxidative stress may play a role in disrupting iron regulation [2]. Furthermore, while many studies characterizing this iron dysregulation have been done in liver, less work has been done to understand the role that iron dysregulation may play in other tissues important to glucose sensitivity such as skeletal muscle. We therefore chose a cell model of C2C12 mouse skeletal muscle cells to characterize oxidative stress-induced iron dysregulation in skeletal muscle.
Curcumin, a naturally occurring compound in the spice, turmeric, has been shown to be effective in improving insulin sensitivity, although the mechanisms by which it does so are not entirely understood [3, 4]. Curcumin has been shown to bind iron and is very likely to have an effect on iron regulation [5]. The purpose of this study is to develop a cell model of oxidative stress-induced iron dysregulation in order to identify the impact that oxidative stress has on iron regulation, and to understand the effects of curcumin on iron regulation and its ability to prevent iron dysregulation.
Methodology
Differentiated C2C12 mouse skeletal muscle cells were treated with H2O2 for 12 hours in normal iron cells. We then developed a high iron cellular model by treating cells with iron for 24 hours. Using this high iron model, we treated cells with H2O2 during the final 12 hours of the iron treatment to understand the effects of oxidative stress on iron regulation in a high-iron environment. We also characterized the effects of curcumin in both normal and high iron cells and examined its ability to prevent this iron dysregulation using a 12-hour curcumin pre-treatment. Following treatments, cells were harvested for protein, iron analysis, and lipid peroxidation assays. Protein levels of the iron intake protein, transferrin receptor (TfR) and iron storage protein, ferritin heavy chain (FHC) were measured using western blot analysis. Total iron was measured using a total iron colorimetric assay.
Results
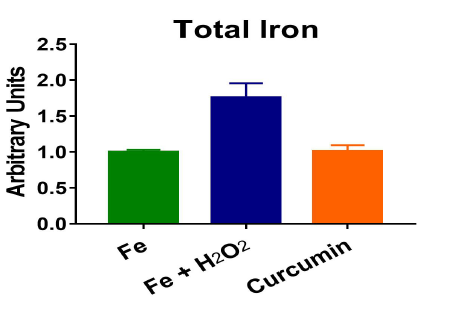
H2O2 treatment in a normal iron model caused an increase in FHC and a decrease in TfR, suggesting that an anti-oxidative stress response was triggered. In our high iron model, the cells had increased levels of total iron, showing that iron treatment successfully loaded iron into the cells. The cells also responded by increasing FHC levels and decreasing TfR levels in an attempt to manage the excess iron. High iron cells treated with H2O2 showed a reversal of iron regulation, with a decrease in FHC and an increase in TfR levels. Despite already having high levels of iron, the H2O2 treatment caused further iron accumulation coupled with less iron storage. Curcumin altered the iron regulation in both normal and high iron conditions. In both normal and high iron cells, curcumin decreased TfR levels. Curcumin also increased FHC levels in high iron cells. Pre-treatment with curcumin prevented iron accumulation (Figure 1) and lipid peroxidation caused by H2O2.
Discussion
We found that the effects of H2O2 on cellular iron regulation were influenced by the existing iron level. While H2O2 seemed to cause an anti-oxidant response in normal iron cells to tighten the regulation of iron, the response was reversed in a high iron model leading to iron dysregulation. These results suggest that there may be a point at which the oxidative stress is un-manageable and caused a
“switch” in the action of iron regulatory proteins, creating a downward spiral of iron dysregulation and oxidative stress. Further work is needed to understand the exact mechanism by which oxidative stress disrupts iron regulation, and where this “switch” occurs in the process. Curcumin was seen to impact iron regulation in skeletal muscle cells and prevented oxidative stress-induced iron dysregulation. Future experiments will aim at understanding the mechanism by which curcumin prevented iron dysregulation, as well as identifying other anti-oxidant compounds that may have similar effects.
Conclusion
Our results show that treatment with H2O2 in the presence of iron causes an accumulation of iron and a decrease in ferritin expression, potentially leading to an increase of free iron and the production of reactive oxygen species. Results also show that curcumin has the potential to prevent iron dysregulation induced by oxidative stress, providing a possible mechanism to its ability to improve insulin sensitivity.
Figure 1: Pre-treatment with curcumin prevented iron accumulation caused by hydrogen peroxide in a high iron model of C2C12 cells.
References
Kunutsor SK, Apekey TA, Walley J, Kain K: Ferritin levels and risk of type 2 diabetes mellitus: an
updated systematic review and meta-analysis of prospective evidence. Diabetes/metabolism research
and reviews 2013, 29(4):308-318.
Bresgen N, Eckl PM: Oxidative stress and the homeodynamics of iron metabolism. Biomolecules 2015,
5(2):808-847.
Na LX, Li Y, Pan HZ, Zhou XL, Sun DJ, Meng M, Li XX, Sun CH: Curcuminoids exert glucoselowering
effect in type 2 diabetes by decreasing serum free fatty acids: a double-blind, placebocontrolled
trial. Mol Nutr Food Res 2013, 57(9):1569-1577.
Chuengsamarn S, Rattanamongkolgul S, Luechapudiporn R, Phisalaphong C, Jirawatnotai S: Curcumin
extract for prevention of type 2 diabetes. Diabetes Care 2012, 35(11):2121-2127.
Hatcher H, Planalp R, Cho J, Torti FM, Torti SV: Curcumin: from ancient medicine to current clinical
trials. Cell Mol Life Sci 2008, 65(11):1631-1652.