Mikel R. Stevens
The objectives of this project:
Our objective was “to develop an understanding of the genetic structure of the varieties within P.
scariosus. With a specific intent to test the hypothesis that P. scariosus var. albifluvis is distinct
within P. scariosus” while mentoring no less than four undergraduate students.
We have meet and exceeded the academic objectives proposed and then funded by this MEG
as attested to in the following paragraphs and attachments to this report. As a result of our most
excellent success in this project we have four additional students who are volunteering on the
project. It is our hope that we can find additional funding to hire at least two of these students.
Mentoring environment:
We have been able to mentor a total of eight undergraduate students while working on this
project. Of those eight, three (Chris Anderson, Kevin Farley, and Nathan Ricks) have worked
long enough to have been coauthors on one published paper directly relating to this project
(Johnson et al., 2016). Two of those three students will also be coauthors with another student
(Chris Anderson, Sarah Harrison, and Nathan Ricks) on two additional peer reviewed
publications. These two additional papers will be submitted within the next few months. We
believe we have the final data collected for these papers and are waiting for the input of the data
from our Ohio State collaborators. The reason we believe we have two papers is that we have
at least two independent stories and data to support each story and the above named three
students have been intimately involved with all aspects of the research.
Six of our mentored students interacting on this project have been coauthors on nine different
presentations at three different national professional meetings and four regional meetings during
2016. Five of the six students have presented twice each at two different meetings. Sarah
Harrison and Nathan Ricks independently received a second place in the poster and oral
competitions, respectively, of the undergraduate division of the American Society for
Horticultural Science in Atlanta, GA in August of this year (see attached). These awards are
rather prestigious in that there are over 20 other undergraduates, from research universities
across the US competing in each of the competitions. All of these presentations and
publications were directly connected with the objectives of this project.
Already published peer reviewed paper with students (see attached):
Johnson, R.L., M.R. Stevens, L.A. Johnson, M.D. Robbins, C.D. Anderson, N.J. Ricks, and K.M.
Farley, 2016. Molecular and morphological evidence for Penstemon luculentus: a new
combination for Penstemon fremontii var. glabrescens. PhytoKeys 63:47-62.
A list of the presentations referred to above:
Anderson, C.D., N.J. Ricks, K.M. Farley, P.J. Maughan, and M.R. Stevens. 2016. Development
of Penstemon scariosus (Plantaginaceae) microsatellite markers. Plant & Animal Genome
XXVI Conference. January 9-13, 2016, San Diego, California. (Poster Presentation).
Anderson, C.D., N.J. Ricks, K.M. Farley, P.J. Maughan, and M.R. Stevens. 2016. Development
and classification of SSRs for Penstemon scariosus (Plantaginaceae). Utah Conference on
Undergraduate Research. February 19, 2016, Salt Lake City, Utah. (Poster Presentation).
Harrison, S., B. Ensign, and M.R. Stevens. 2016. Interspecific hybrids in Penstemon. Utah
Conference on Undergraduate Research. February 19, 2016, Salt Lake City, Utah. (Poster
Presentation).
Harrison, S., B. Ensign, and M.R. Stevens. 2016. Interspecific hybridization
within Penstemon and their Potential uses in urban landscapes. American Society for
Horticultural Science Annual Conference, August 8-11, 2016, Atlanta, Georgia. (Poster
Presentation).
Ricks, N.J., M.R. Stevens, R.L. Johnson, L.A. Johnson, C.D. Anderson, M.D. Robbins, and K.M.
Farley. 2016. The development and use of SSR markers for Penstemon scariosus, a
species with horticultural potential. American Society for Horticultural Science Annual
Conference, August 8-11, 2016, Atlanta, Georgia. (Oral Presentation).
Rodriguez-Pena, R.A., A.D. Wolfe, M.D. Robbins, R.L. Johnson, L.A. Johnson, C.D. Anderson,
N.J. Ricks, K.M. Farley, and M.R. Stevens. 2016. Population genetics and geographic
patterns among varieties of Penstemon scariosus. Botanical Society of America Annual
Conference, Botany 2016, July 30 – August 3, 2016, Savannah, Georgia. (Oral
Presentation).
Stevens, M.R., R.L. Johnson, L.A. Johnson, M.D. Robbins, C.D. Anderson, N.J. Ricks, and K.M.
Farley. 2016. Unraveling the Penstemon scariosus complex using molecular markers – an
update. Annual Utah Rare Plant Meeting. March 8, 2016. Salt Lake City, Utah. (Oral
Presentation).
Stevens, M.R., S. Harrison, B. Ensign, A.D., Robbins, M.D., Johnson, Robert, L.A, Johnson,
C.D., Anderson, N.J., Ricks, and K.M., Farley. 2016. Breeding native flowers for drought
tolerant urban landscapes: 2016 progress report. Annual WERA Meetings, October 6-8,
2016, Ames, Iowa. (Oral Presentation).
Budget:
The MEG of $20,000 has been spent in approximately the following categories.
Student wages ~$13,000.00
Travel to take students to meetings* ~$3,500.00
Supplies ~$3,500.00
Total $20,000.00
*This budget assisted in taking the students to two national meetings
1- Plant & Animal Genome XXVI Conference. January 9-13, 2016, San Diego, California. Where
Chris Anderson and Kevin Farley presented their poster (described above).
2- American Society for Horticultural Science Annual Conference, August 8-11, 2016, Atlanta,
Georgia. Where Sarah Harrison and Nathan Ricks presented their respective poster and oral
presentations. Each placing 2nd in their respective competitions (see attached certificates).
The following summary of our studies, to date, has almost exclusively been accomplished by
the undergraduate students Chris Anderson, Kevin Farley, Sarah Harrison, and Nathan Ricks.
A summary of our studies of the Molecular Characterization of Penstemon scariosus (Plantaginaceae)
Abstract:
This summary report is of what we have, and are still learning regarding Penstemon scariosus,
with a focus on the variety albifluvis. Using our molecular markers developed for this study we
found evidence that the traditional P. scariosus var. albifluvis may need to be returned to its
original species taxonomic designation of P. albifluvis. It also must be pointed out that the
broader Penstemon scariosus study is still on going. During the 2015 year of analyzing our data
we discovered a putative P. scariosus population near Tabiona, Utah which was clearly unique
by both measuring its morphological characteristics and with our molecular markers. Because
we were so unsure of that unusual find we deliberately withheld those samples from the
remaining samples used to arrive at the study results we are reporting here. Initially, we were
concerned that there was an error in with the Tabiona samples. During the 2016 field season we
returned to that region and collected well over an additional dozen sample locations and also
collected additional population locations of P. gibbensii from across Wyoming and Colorado to
assist us in our understanding of the Tabiona genotype in relationship to P. scariosus complex.
These additional samples are being molecularly and statistically analyzed now. We believe we
will be able to develop a scientifically peer reviewed paper later this year or early next year
which will discuss the results reported here in context with what we are discovering now. During
this study we also found that P. fremontii var. glabrescens should be a distinct species which we
named P. luculentus.
Introduction:
Noel Holmgren and others have stated that “P. scariosus exhibits a complex range of variability”
(Holmgren, 1984; Neese and Atwood, 2008). Although, Neese and Atwood (2008) stated that
variety albifluvis is more distinct than the three other reported members of this species.
Curiously, it was originally described as a distinct species (England, 1982); however, in 1984
Noel Holmgren listed it as a variety of P. scariosus (Holmgren, 1984). Variety albifluvis is found
almost exclusively on the oil shale ledges of the Green River Formation. In the last few years,
there has been an ever increasing interest in recovering the oil found in those formations
(Robinson, 2007). Because of its unique limited habit and the increasing interest to recover the
hydrocarbons found in this Green River Formation it was considered a candidate species for
listing as an endangered species under the Endangered Species Act of 1973 (Ashe, 2013). To
illustrate the potential clash between the oil shale recovery efforts and the preservation of P.
scariosus var. albifluvis one only need to drive along Dragon Road south of Bonanza, UT (see
photos of the exact opposite sides of the dirt road, Fig. 1A&B).
Collecting Penstemon for Study:
Early summer 2013 we initiated a study of P. scariosus by sampling tissue of eight unique
plants from multiple locations across the range of the species (see Fig. 2). Early spring 2014 we
searched multiple herbarium databases where we found records suggesting that P. scariosus
geographic range was mildly larger than we had previously thought. In the Brigham Young
University S. L. Welsh Herbarium samples, we found several curious Penstemon specimens
labeled as P. scariosus. These specimens were from Piceance Basin, Colorado. The specimens
were unusual for a couple of reasons. First, they were somewhat outside the well-documented
range of P. scariosus; and, second, although they keyed out to P. scariosus using A Utah Flora,
the specimens had hirtellous stems, a trait not found in P. scariosus. These observations were
enough to have us include the Piceance Canyon region in our planned collections. We
concluded that if these plants were indeed part of the P. scariosus complex they needed better
characterization.
In addition to the unique Piceance Canyon population we also found several other populations
of P. scariosus in Wyoming which we were unaware of. However, one new record of a P.
scariosus population was unusual in that it was reported to be on the Book Cliffs ridge in Grand
County, Utah. These old herbarium records were about 20 miles south of all known P. scariosus
var. albifluvis populations. Furthermore, there were no other records of any other P. scariosus in
over 50 miles of this putative remote population. Late June 2014 we collected one reblooming
sample from this population and it keyed out as P. scariosus var. albifluvis.
We essentially completed our P. scariosus complex sample collections late spring and early
summer 2014. We were assisted with the collections of P. scariosus var. albifluvis by individuals
connected with the BLM Vernal, UT office. In total, we collected material from 17 field locations
of P. scariosus var. albifluvis, 8 of P. scariosus var. cyanomontanus, 25 of var. garrettii, 9 of var.
scariosus, and 11 locations of the unusual Penstemon found in Piceance Canyon, Colorado.
To gain an improved understanding of the extent of the Book Cliff P. scariosus var. albifluvis
population(s) we returned to that location in early June 2015. Following which we continued
searching, wherever legally possible, for additional remote locations of this taxon across the
entire range of the Book Cliffs. We were able to conduct an extensive survey along the Utah
and Colorado Book Cliffs ridge for P. scariosus with the assistance of four BYU undergraduates
and a small grant from Uinta County, Utah and Rio Blanco County, Colorado. In our search we
found that the Book Cliffs P. scariosus var. albifluvis population extended along the ridge in
Grand County, Utah, mostly on southern exposures with a few plants scattered on the very top
of the ridge for approximately three air miles. Thousands of plants were found in a narrow band
(from a few feet wide to upwards to ~100 ft. at the widest point) along a Green River shale
geological formation for that distance. This geology is very similar to where this taxon has
historically been found to occur, at lower elevations closer to the White River. We did not expect
the population on the Book Cliffs to be so extensive. However, that was our only discovery of
new/expanded P. scariosus var. albifluvis populations. For the remaining four days we
searched, with no avail, on accessible sites following the Book Cliffs to their eastern terminus
north of Rifle, Colorado. The only population of P. scariosus var. albifluvis encountered
remained those already described above. The results of our search does not mean that there
are no new populations to be discovered in this region. There may be populations on private
land or tribal land where we were unable to access or on difficult to access public lands.
However, it should also be noted that we did find habitat that looked to be ideal for P. scariosus
var. albifluvis in several locations but when searched there were no plants found.
Clues of a Misclassified Penstemon:
After studying all of our Piceance Canyon samples morphology we realized that it was indeed
unique compared to P. scarious. Furthermore, we learned that it had already been described as
P. fremontii var. glabrescens (Dorn and Lichvar, 1990). However, this clarification came as a
surprise in that we found P. fremontii var. fremontii within less than 100 yards of populations of
variety glabrescens in Piceance Canyon. We were never able to locate any identifiable hybrids
between the two taxa and they were easily distinguishable by their morphological
characteristics. Moreover, their overall morphology reminded us more of P. scariosus, which is
why it is not surprising that the BYU herbarium samples were identified as P. scariosus by their
collectors rather than a variety of P. fremontii. Thus, with all these discoveries in mind we
concluded that this taxon needed better characterization. Consequently, we decided to utilize
our molecular tools to study this taxon (P. fremontii var. glabrescens) along with our P.
scariosus samples.
Penstemon DNA “Fingerprinting”:
To best explain how we approached the DNA molecular studies of our samples we will use the
analogy of “fingerprinting.” That is, like human fingerprints, each individual plant has its own
unique DNA “fingerprint” which can be studied. However, this “fingerprinting” analogy breaks
down when we learn that it is impossible to tell who the parents of person are by comparing the
fingerprint of a child to that of her parents, because, a fingerprint pattern is not inheritable. There
simply is not a way to identify a family relationship by comparing the parents and their child’s
fingerprints. On the other hand, we can readily identify genetic relationships using DNA since
we inherit half of our DNA “fingerprint” from our mother and the other half from our father.
Therefore, our unique DNA “fingerprint” is an exclusive combination of half of our mothers’ DNA
“fingerprint” and half or our fathers molecular “fingerprint.”
The DNA “fingerprinting” technology we choose to develop and use in our P. scariosus study
(Anderson et al., 2016; Johnson et al., 2016) is the same methodology used by the court system
which can precisely demonstrate that the person was the perpetrator of a crime. It is also the
same method that can be used to determine the paternity (father) of a person. There are two
names which are used for this common molecular “fingerprinting” methodology, one name is,
simple sequence repeats (SSRs), and the other name is “microsatellites.” Each SSR
(microsatellite) is a short DNA sequence found at a reliably specific location on a chromosome.
The way we identify any given microsatellite, without error, is using a molecular biology
laboratory procedure called a PCR (polymerase chain reaction). When we use the PCR
procedure under the correct conditions, the resulting DNA fingerprints are relatively quickly
deciphered when interpreted by someone trained in the field.
The first question we addressed in our DNA fingerprinting studies was the suspicious
relationship between P. fremontii var. fremontii and variety glabrescens. Finding these two taxa,
living within yards of each other, with no apparent hybrids between the two, as well as being
able to readily morphologically distinguish between them, allowed us to set up a testable
scientific hypothesis.
Study of the P. fremontii Varieties:
We learned that there is not a close genetic relationship between P. fremontii var. fremontii and
var. glabrescens. Or for that matter, P. fremontii var. glabrescens is not closely related to any
other suspected Penstemon of the region. Using our SSR fingerprint data as support, as well as
our morphological observations, we concluded that this taxon should be considered a species in
its own right. We presented the statistical results of all of our molecular studies, as well as a
map, and related background information regarding the redefinition of this interesting taxon in a
recently publish paper (Johnson et al., 2016). Because the name P. glabrescens has already
been used for a Penstemon in southern Colorado and northern New Mexico we cannot elevate
the variety name to a species for this taxon’s name (Pennell, 1920). Therefore, we renamed it P.
luculentus. This name is derived from the Latin word for “luculentus,” meaning brilliant or bright.
The name was chosen to reflect the brilliant blue flower color, which is particularly striking in the
field in contrast to the whitish or tan shale background typically associated with this species
(Fig. 3A&B).
Results of the Study of the P. scariosus Complex:
Once we determined that P. fremontii var. glabrescens needed to be described as a distinct
species we turned our attention to understand how various populations of the P. scariosus
complex were related to each other. Since the majority of our funding focus was on improving
our understanding of the genetic diversity of P. scariosus var. albifluvis we secured samples at
more sites across a smaller geographic range than the other members of the P. scariosus
complex. However, our study did include samples from the known perimeter of P. scariosus
along with samples interlaced throughout its range (Table 1; Fig. 2, 4, and 5).
Once securing our tissue samples we initiated the molecular and data analysis aspects of the
study. We found that there were no clear delineations between varieties cyanomontanus,
garrettii, and scariosus. That is, using the SSRs molecular markers, we could not find distinctive
genetic population alignments with the present variety definitions with any sort of statistical
confidence and the recognitions of var. cyanomontanus is questionable. Our data clearly agree
with what Holmgren (1984) specifically suggested about distinguishing a variety with
cyanomontanus morphological characteristics as a taxon was questionable. However, the SSR
marker results clearly suggest that P. scariosus var. albifluvis is statistically more distinct from
the rest of the P. scariosus complex. Our results also suggest that its closest relative may be P.
scariosus accessions north of Roosevelt, Utah (Fig. 2 [sample #36]). Nevertheless, it is rather
distinct compared to the rest of P. scariosus. However, even with these finding being so clear
we are collaborating with Andi Wolfe, a recognized Penstemon authority from Ohio State
University, to evaluate these same samples with a much more comprehensive molecular marker
technique to see if these new test collaborate our results. When we complete both molecular
testing methods, we are working on now we will prepare one, or more, manuscript(s) for peer
review and publication in reputable scientific journal(s). It should be pointed out that in 2016 we
collected very compelling evidence that there may be a new taxon that has traditionally been
classified as either var. garrettii or var. scariosus in the region of Tabiona, UT. We have now
collected many samples from a number of populations of these unusual Penstemon and their
data will be included in future analysis of Penstemon scariosus and publications of those
results.
To better visualize what we learned about the P. scariosus complex from our SSR marker data
which we collected from 2013-15 we have created a map where each P. scariosus collection
location is represented as a pie chart of the percent of shared, or distinctive aspects of their
genetic relationships (Fig. 2). When comparing all of our samples to the presently named four
varieties of P. scariosus we can statistically identify three related “groups” with significantly
different genetic “fingerprints.” We assigned a color to each of those three groups (red, green,
and blue [Fig. 2]). Using this visualization method, it becomes evident when studying this map
that P. scariosus var. albifluvis is distinctive (the mostly green pie charts [Fig. 2]), both with its
molecular fingerprint and its geographic isolation. We again performed the same statistical
method (STRUCTURE) analyzing strictly the P. scariosus var. albifluvis accessions (Fig. 4) and
a separate STRUCTURE analysis of the remaining P. scariosus samples (Fig. 5). The results of
those analysis assisted us in “teasing out” a more refined understanding of the population
genetic structures of the non P. scariosus var. albifluvis samples collected in 2013-15.
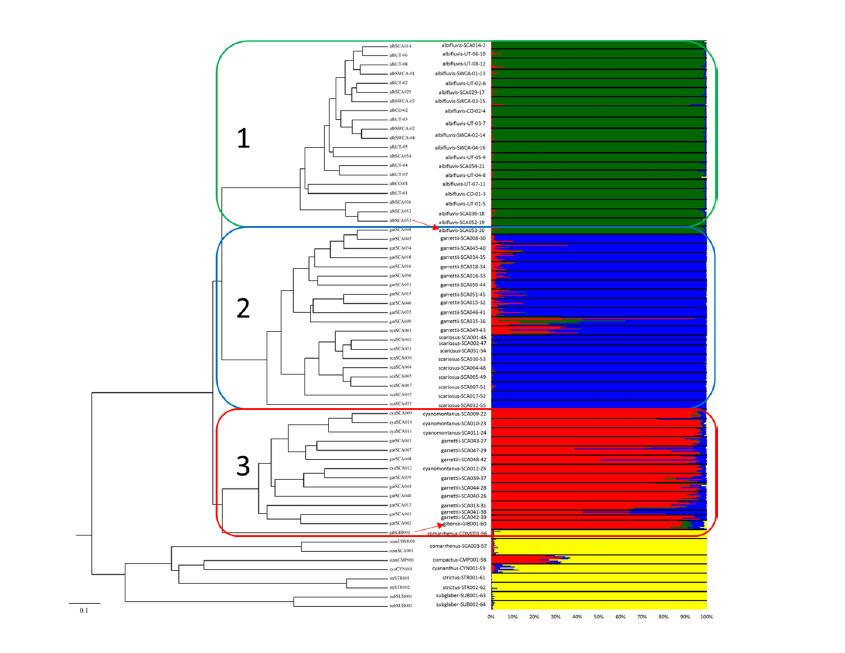
The dendrogram (Fig. 6) of our preliminary analysis clearly suggest that all varieties of P.
scariosus and P. gibbensii are related to each other. Using the data that we have generated and
analyzed thus far suggest that var. albifluvis is most closely related to var. scariosus and the
southwestern accessions of var. garrettii. Variety albifluvis is more distantly related to the more
eastern accessions of var. garrettii, all of var. cyanomontanus and P. gibbensii (Table 1 and Fig.
6). Additionally, our data suggest that there is a lower level of genetic diversity within var.
albifluvis than the amount of diversity found within and between the three remaining putative
varieties of P. scariosus (Fig. 2, 5, and 6).
We are in the midst of a study of revisiting the question of how to use morphological
characteristics to see if we can accurately distinguish between the historically defined remaining
three varieties of the P. scariosus complex. If we are successful, in finding more definitive, than
the presently used morphological plant characteristics, it would allow us to recommend a
revision of the descriptions of the apparent varieties within P. scariosus. Our objective is to find
morphological characteristics that more accurately reflects the results of our molecular study.
Finally, we should report on the identification of the unusual accessions we have collected in
2016 near Tabiona, UT. These samples are identified in the field by the fact that mature plants
are rather robust, both in their leaf, and flower size, compared to the var. garrettii of the region.
Because the “unknown” does key out to be P. scariosus it is a prominent hypothesis of ours that
it may indeed be a member of P. scariosus; however, if it is a P. scariosus, it may be
independent of all presently identified members of the species. We have collected over 15
accessions of this “unknown” Penstemon from the Red Creek area east of Fruitland on the
southwestern corner to the community of Strawberry, UT on the southeastern corner to several
miles east of Tabiona on northeastern corner and up all the canyons surrounding both Tabiona
and Hanna, UT on the northwestern corner. At this time, we are unclear as to the true edges of
this “unknown.” All of the “unknown” samples have a very similar morphological appearance and
a molecular marker fingerprint. We are working on the further understanding of the uniqueness
of this new “unknown” Penstemon. We believe that the reason for this new “unknown” being
previously overlooked is twofold. First, it clearly keys taxonomically out to be P. scariosus and
second, it appears to be a very narrow endemic of the geography described above.
Conclusion:
Using multiple herbarium records, we were able to delineate where P. scariosus has been found
historically. We drove thousands of miles and walked for many hours collecting samples from
over 70 locations for genetic comparison. The study of the herbarium records and field samples
led to the discovery of an important population of P. scariosus var. albifluvis, a problem with the
classification P. fremontii var. glabrescens that suggested a need to be more carefully studied,
and the discovery of a potentially new “unknown” Penstemon taxon. For this study we
developed a special set of Penstemon SSR markers to study P. scariosus and P. fremontii DNA
fingerprints which we published in a peer reviewed journal using solely undergraduate students
(Anderson et al., 2016). Using these markers we found evidence that the traditional P. scariosus
var. albifluvis may need best be treated as its original taxonomic designation of P. albifluvis
(England, 1982). We also found that P. fremontii var. glabrescens (Dorn and Lichvar, 1990) was
a distinct species which we named P. luculentus. We accomplished that study and published
that peer reviewed article with the same undergraduates (Johnson et al., 2016). We are now
working to identify how P. scariosus, P. albifluvis, P. gibbensii, and the new “unknown”
Penstemon are all related to each other.
References:
Anderson, C.D., Ricks, N.J., Farley, K.M., Maughan, P.J., and Stevens, M.R., 2016.
Identification and characterization of microsatellite markers in Penstemon scariosus
(Plantaginaceae). Appl Plant Sci 4:1500105.
Ashe, D.M., 2013. Endangered and threatened wildlife and plants; threatened species status for
Graham’s beardtongue (Penstemon grahamii) and White River beardtongue
(Penstemon scariosus var. albifluvis), p. 47590–47611, Federal Register. Department of
the Interior Fish and Wildlife Service.
Dorn, R.D. and Lichvar, R.W., 1990. A new variety of Penstemon fremontii (Scrophulariaceae)
from Colorado. Madroño: A West American Journal of Botany 37:195–199.
England, J.L., 1982. A new species of Penstemon (Scrophulariaceae) from the Uinta Basin of
Utah and Colorado. Gt. Basin Nat. 42:367–368.
Holmgren, N.H., 1984. Penstemon, p. 370–457. In: Cronquist, A., Holmgren, A. H., Holmgren,
N. H., Reveal, J. L., and Holmgren, P. K. (eds.), ain West. New York Botanical Garden,
Bronx, NY.
Johnson, R.L., Stevens, M.R., Johnson, L.A., Robbins, M.D., Anderson, C.D., Ricks, N.J., and
Farley, K.M., 2016. Molecular and morphological evidence for Penstemon luculentus: a
new combination for Penstemon fremontii var. glabrescens. PhytoKeys 63:47–62.
Neese, E.C. and Atwood, N.D., 2008. Penstemon Mitchell, p. 702–733. In: Welsh, S. L.,
Atwood, N. D., Goodrich, S., and Higgins, L. C. (eds.), A Utah Flora. Printing Services,
Brigham Young University, Provo, UT.
Pennell, F.W., 1920. Scrophulariaceae of the central Rocky Mountain states. Contr. U.S. Natl.
Herb. 20:313–381.
Robinson, E., 2007. Colorado’s imperiled Penstemon. Bul. Amer. Penstemon Soc. 66:2–9.
Figure 2. This map is of the northeastern corner of Utah and adjacent areas in Wyoming and
Colorado (note the US location in lower left panel of the figure). The individual colored pie charts
are where our 2013-2015 sample collections were made. These collections also represent the
reported range of what has been described as Penstemon scariosus. The colors of the pie
charts represent the percent of genetic diversity which we found in our study. The region
outlined in black is considered to be where P. scariosus var. albifluvis is to be found. The region
outlined in green is considered to be where P. scariosus var. cyanomontanus is to be found.
The region outlined in blue is considered to be where P. scariosus var. garrettii is to be found.
The region outlined in red is considered to be where P. scariosus var. scariosus is to be found.
The key to each accession sample number is found in Table 1. Note that the green pie charts
are essentially geographically isolated from all other P. scariosus.
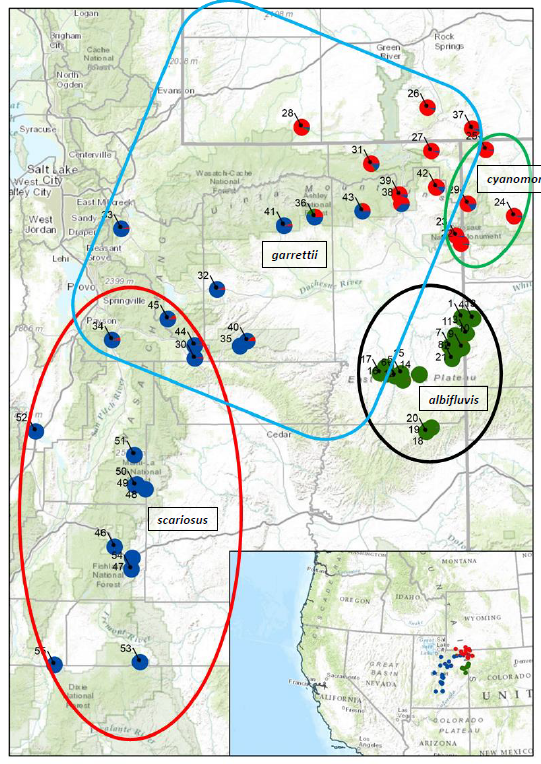


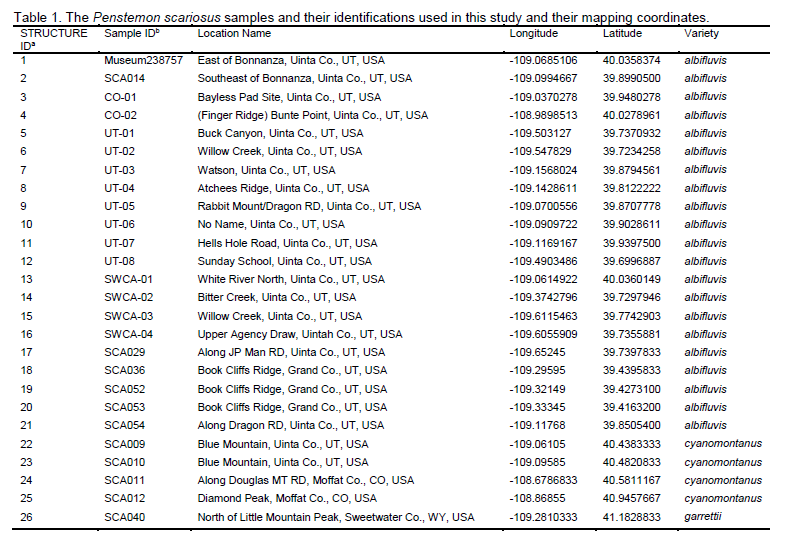


Figure 4. This map is of the region of the Uinta Basin of Utah and adjacent area of Colorado (it
is the expanded area of the green pie charts found in Fig. 2). The individual colored pie charts
are where the samples of P. scariosus var. albifluvis were made. These collections also
represent the reported range of what has been described as Penstemon scariosus var.
albifluvis. The colors of the pie charts represent the percent of genetic diversity which we found
within only var. albifluvis in our study. Note that the green and red pie charts are essentially
scattered across region. These preliminary data are suggesting that there are no real genetically
unique populations of P. scariosus var. albifluvis. The key to each accession sample number is
found in Table 1.
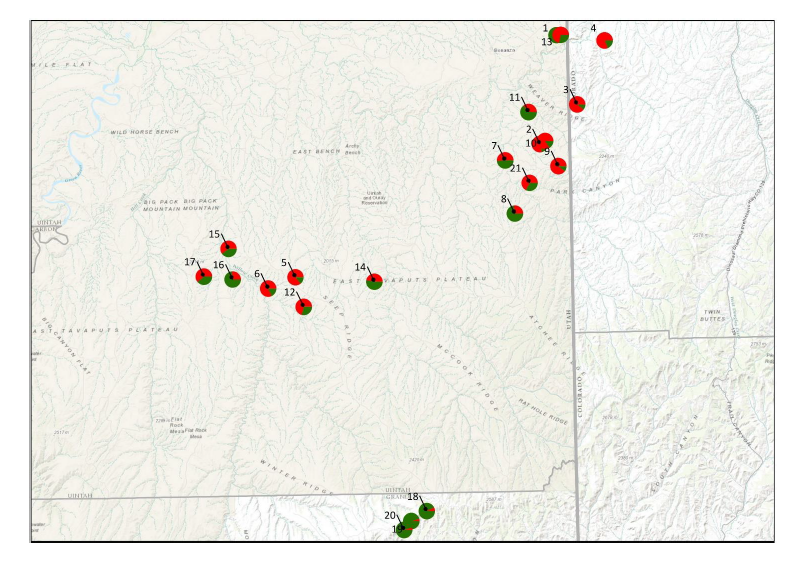
Figure 5. Essentially this map is the same as Fig. 2, except it is missing the P. scariosus var.
albifluvis sample accessions. This map focuses on the genetic diversity found in the accessions
collected of the traditionally described as P. scariosus var. cyanomontanus, var. garrettii and
var. scariosus. These collections also represent the reported range of these varieties of
Penstemon scariosus. The colors of the pie charts represent the percent of genetic diversity
which we found within and between these taxa. Note that the color distribution of these pie
charts do represent more closely the traditional geographic regions of the varieties of P.
scariosus which are reported by Holmgren (1984) and Neese and Atwood (2008). The key to
each accession sample number is found in Table 1.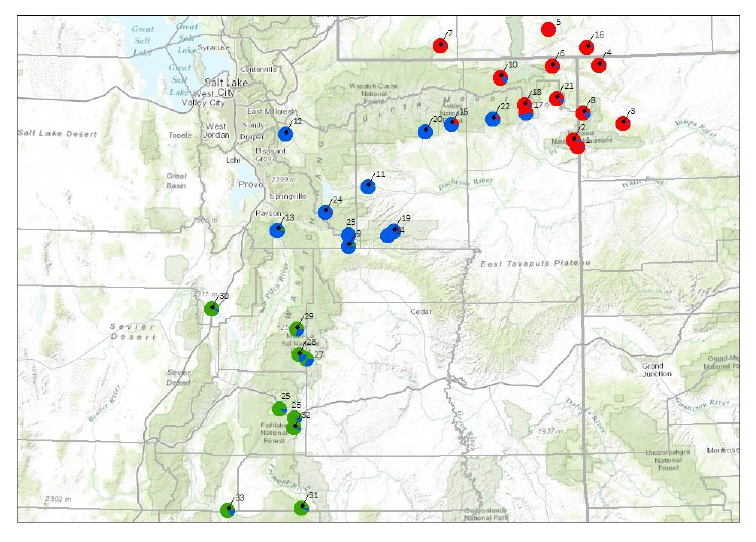
Figure 6.
The key to each Penstemon scariosus accession sample represented in this dendrogram is
found in Table 1. There are three “boxed” sections of this dendrogram. Box 1 (the green box)
represent all, and only, the accessions collected of var. albifluvis (see Fig. 2, and 4). Box 2 (the
blue box) represents the samples of P. scariosus from the southern portion of the range (see
Fig. 2, and 4) of this species with includes all of traditionally classified as var. scariosus and the
southern portion of those classified as var. garrettii. Finally, Box 3 (the red box) includes the
north eastern accessions (see Fig. 2, and 4) of the traditionally classified var. garrettii, all of var.
cyanomontanus and it includes our one sample of P. gibbensii.