Garrett Hamblin and Faculty Mentor: Scott Weber, Department of Microbiology and Molecular Biology
Properly functioning helper T cells are crucial in a response to an infection. The adaptive immune response is orchestrated by T helper cells and their function is dependent upon interactions between the T cell receptor (TCR), peptide MHC (pMHC) and co-receptors. Upon TCR interaction with a foreign antigen, a calcium signaling cascade is initiated, which determines T cell activation, survival, proliferation and differentiation. CD5 is a T cell co-receptor that is a negative regulator of T cell activation. T cells with higher CD5 expression respond better to foreign antigen than those with lower CD5 expression. Cell-surface expression of CD5 is proportional to T cell receptor signaling capacity for self-peptide. Because of this, CD5 acts to fine-tune the T cell response. Treatment with CD5 monoclonal antibodies causes increased intracellular Ca2+ in T cells.1 In our study, we examined the role of CD5 expression and calcium signaling in the T cell response to foreign antigen. To shed further light on this, we evaluated two helper T cells from T cell receptor transgenic mice (LLO118 and LLO56) that differ in primary and secondary response to a peptide of Listeria monocytogenes.2 They are also known to differ in CD5 levels.2 We found that each T cell has unique calcium mobilization in response to in vitro stimulation and that CD5 expression levels in these cells changed over time following stimulation. To further evaluate the role of CD5, we measured calcium signaling in CD5 knockout LLO118 and LLO56 T cells at three time points (naïve, day 3, day 8) and found that CD5 plays a significant role in promoting the calcium signaling of naïve CD5-high LLO56 T cells. LLO56, LLO118, and CD5 knockout LLO56 and LLO118 mice were bred and housed in pathogen free conditions. All mice used were 5–12 weeks old. All use of lab animals was done with approval of ACUC at BYU. Splenocytes from each type of mice were isolated and stimulated at three time points (naïve, 3rd day, and 8th day). Calcium flow cytometry was done to analyze the alteration of intracellular calcium concentrations in the T cells. This method allowed for identification and analysis of cells within a population based on their light-scatter profile and selective responsiveness to specific stimuli. To identify the T helper population, cells were labeled with fluorochrome-conjugated antibodies specific to mouse antigens. In our case, we selected the T helper cell population. Flou-4, a labeled calcium indicator that exhibits an increase in fluorescence upon binding to Ca2+, was used to measure the difference in signal levels.
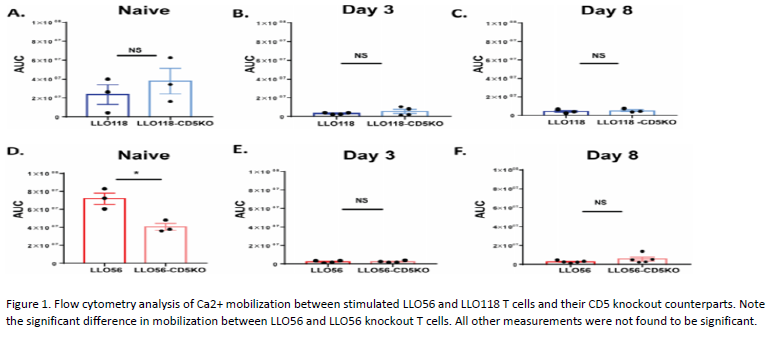
LLO56 naive T helper cells showed higher CD5 expression than naïve LLO118 T helper cells. However, upon stimulation, the CD5 expression differences between LLO56 and LLO118 T cells decrease over the course of 8 days of stimulation. To further investigate the role CD5 expression plays in Ca2+ mobilization, we measured the calcium signal in T cells from LLO118-CD5 knockout and LLO56-CD5 knockout mice. We found in the LLO118 T cells (CD5-low) that calcium mobilization was not significantly different from LLO118-CD5 knockout T cells at any of the three time points (Fig 1A–1C). Conversely, naïve LLO56-CD5 knockout T helper cells had significantly lower calcium levels compared to the naïve LLO56 T cells (CD5-high) (Fig 1D). There was no calcium mobilization difference seen at day 3 or day 8 post-stimulation (Fig 1E and 1F). Thus, in naïve LLO118 T cells (CD5-low), CD5 does not play a strong role in regulating calcium mobilization at any of the time points. However, CD5 expression is important in regulating calcium mobilization in the naïve LLO56 T cells (CD5-high) during the initial response to antigen. As CD5 levels decrease over time, its role in regulating calcium also decreases.
In this study, we examined the role of CD5 in regulating T cell activation using two T helper cells, LLO56 and LLO118, which bind to the same L. monocytogenes epitope and have different levels of CD5 on the surface upon completion of thymic development. The distinct Ca2+ mobilization patterns of LLO56 and LLO118 likely influence their particular responses to antigen. Here we characterized the effect of CD5 on calcium mobilization in CD5-high and CD5-low T cells over the course of 8 days. We found that naïve LLO56 T helper cells (CD5-high) have significantly higher calcium mobilization compared to the LLO56-CD5 knockout T cells, but at later time points the removal of CD5 did not significantly alter LLO56 calcium mobilization. Naïve LLO118 T helper cells (CD5-low) exhibit no differences in Ca2+ mobilization relative to their CD5 knockout counterpart. Thus, we found naive CD5-high T cells have improved calcium mobilization to an antigen they have never seen before and CD5 expression plays an important role in intracellular Ca2+ mobilization for naïve LLO56 helper T cells (CD5-high).
CD5-high cells have better basal TCR signaling and improved functional characteristics which correlate with better response to foreign peptide.3 T cells with high CD5 expression are enriched in memory cell populations, suggesting that CD5 levels and self-peptide and foreign peptide interactions are an important consideration when designing vaccines.4 The data presented here helps to elucidate the role CD5 plays in regulating calcium signaling in naïve cells after cell activation during in vitro primary response.
1. June, C.H., P.S. Rabinovitch, and J.A. Ledbetter, CD5 ANTIBODIES INCREASE INTRACELLULAR IONIZED CALCIUMCONCENTRATION
IN T-CELLS. Journal of Immunology, 1987. 138(9).
2. Weber, K.S., et al., Distinct CD4+ helper T cells involved in primary and secondary responses to infection. Proc Natl Acad Sci
U S A, 2012. 109(24): p. 9511-6.
3. Hogquist KA, Jameson SC. The self-obsession of T cells: how TCR signaling thresholds affect fate decisions in the thymus and
effector function in the periphery. Nature immunology. 2014; 15(9):815–23. https://doi.org/10.1038/ni.2938
4. Mandl JN, Monteiro JP, Vrisekoop N, Germain RN. T cell-positive selection uses self-ligand binding strength to optimize
repertoire recognition of foreign antigens. Immunity. 2013; 38(2). Epub 2013/01/08. https://doi.org/10.1016/j.immuni.2012.09.011
