Aaron Leifer and Faculty Mentor Jeffery Tessem, Nutrition, Dietetics, and Food Science
Project Purpose: Our goal is to determine if MafB is necessary for β Cell proliferation and glucose stimulated insulin secretion (GSIS).
Project Importance: Approximately 29.1 million people are affected by type 1 or type 2 diabetes in the United States, according to 2014 National Diabetes Statistic Report. In addition, these 29.1 spent or lost 245 billion dollars combined. A cure for diabetes would result in not only a better life style but would also save billions of dollars.
Project Profile Body: Both forms of diabetes are ultimately caused by decreased functional β-cell mass. β-cell transplantation is a possible treatment for people with diabetes, however there is a lack of β-cell donors. A potential solution is to expand β-cells ex vivo to be used for transplantation. Thus, defining the pathways that allow β-cells to replicate, while still being able to secrete insulin in response to elevated blood glucose, is essential. The Tessem lab is focused on defining the molecular mechanisms that allow β-cells to replicate while maintaining or improving β- cell survival and function.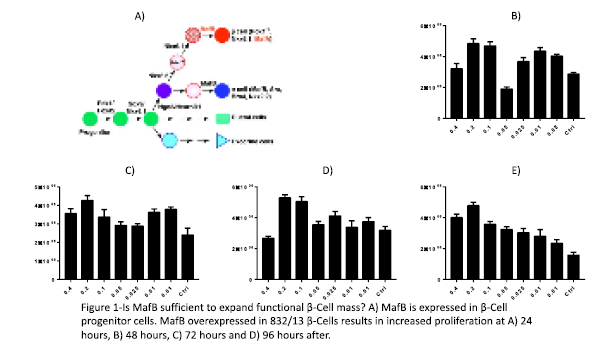 β-cell proliferation is greatest in utero before stage E18.5[1]. MafB is temporarily expressed in β-cell progenitors during in utero β-cell expansion. The time period when MafB is highly expressed in β-cells, corresponds with β- cell proliferation and increased ability to secrete insulin. Interestingly, MafB expression rapidly drops after E18.5[2]. After downregulation of MafB, the β-cell begins to express MafA, Pdx1, and Nkx6.1 (Figure 1A). The Tessem lab and others, have explored the effects of inducing expression of these transcription factors in mature β-cells on proliferation, survival, and insulin secretion. Dr. Tessem showed that the transcription factor Nkx6.1 increases β-cell proliferation, survival and function.[3]. Other studies have shown that Pdx1 increases β-cell proliferation[4]. Based on these previous studies we hypothesize that MafB overexpression in mature β-cells will increase the number of functional β-cells. We have begun studying this process by determining if MafB overexpression increases INS-1 832/13 β-cell proliferation rates. Our preliminary data using varying concentrations of MafB demonstrates that proliferation is increased at 24 (Fig. 1B), 48 (Fig. 1C) 72 (Fig 1D) and 96 hours (Fig 1E) after adenoviral transduction, with the greatest increase observed between 72 to 96 hours after after MafB introduction. In this study we will test if MafB increases functional β-cell mass by measuring β-cell proliferation, survival and insulin secretion. We will use adenovirus that express MafB (AdCMV-MafB, experimental condition) or GFP (AdCMV-GFP, negative control). We will use the insulin producing INS-1 832/13 β-cells and will compare cells expressing MafB and GFP to untreated cells (second negative control). We will complete our proliferation measurements using cell count assays, as well as measuring cellular fitness through the Alamar Blue, MTT and Mitrotracker assays. This will
β-cell proliferation is greatest in utero before stage E18.5[1]. MafB is temporarily expressed in β-cell progenitors during in utero β-cell expansion. The time period when MafB is highly expressed in β-cells, corresponds with β- cell proliferation and increased ability to secrete insulin. Interestingly, MafB expression rapidly drops after E18.5[2]. After downregulation of MafB, the β-cell begins to express MafA, Pdx1, and Nkx6.1 (Figure 1A). The Tessem lab and others, have explored the effects of inducing expression of these transcription factors in mature β-cells on proliferation, survival, and insulin secretion. Dr. Tessem showed that the transcription factor Nkx6.1 increases β-cell proliferation, survival and function.[3]. Other studies have shown that Pdx1 increases β-cell proliferation[4]. Based on these previous studies we hypothesize that MafB overexpression in mature β-cells will increase the number of functional β-cells. We have begun studying this process by determining if MafB overexpression increases INS-1 832/13 β-cell proliferation rates. Our preliminary data using varying concentrations of MafB demonstrates that proliferation is increased at 24 (Fig. 1B), 48 (Fig. 1C) 72 (Fig 1D) and 96 hours (Fig 1E) after adenoviral transduction, with the greatest increase observed between 72 to 96 hours after after MafB introduction. In this study we will test if MafB increases functional β-cell mass by measuring β-cell proliferation, survival and insulin secretion. We will use adenovirus that express MafB (AdCMV-MafB, experimental condition) or GFP (AdCMV-GFP, negative control). We will use the insulin producing INS-1 832/13 β-cells and will compare cells expressing MafB and GFP to untreated cells (second negative control). We will complete our proliferation measurements using cell count assays, as well as measuring cellular fitness through the Alamar Blue, MTT and Mitrotracker assays. This will
demonstrate if MafB is sufficient to enhance β-cell proliferation (which our preliminary data suggests). We hypothesize that MafB will increase INS-1 832/13 β-cell proliferation. Second, we will measure the ability of MafB overexpression to protect β-cells from apoptotic stimuli. We have shown that culturing β-cells with 1mM palmitate induces apoptosis. We will compare INS-1 832/13 β-cells that are untreated with those expressing GFP or MafB. Cells will be transfected, cultured for 72 hours, and then exposed to 1mM palmitate for 24 hours. Cell viability will be measured by cell counts, alamar blue, MTT and cleaved caspase 3 assays. We hypothesize that MafB will enhance cell survival. We will also measure the effect of MafB overexpression on INS-1 832/13 β-cell insulin secretion. Untreated INS-1 832/13 β-cells will be compared to cells expressing GFP or MafB. Insulin secretion will be measured by culturing INS-1 832/13 β-cells in the presence of 2.5mM and 16.7mM glucose and measuring insulin secretion with an insulin radioimmunoassay. We hypothesize that MafB will increase the ability to secrete insulin. Each experiment will be conducted a minimum of 3 times to ensure accuracy. Statistical analysis will be completed using the program GraphPad Prism. Statistical significance will be determined using the student t-test or ANOVA (where appropriate) and significance will be determined using a p-value of less than 0.05.
Project Results: We repeated the proliferation test, three times, at 96 hours after applying adenovirus and were able to confirm that our preliminary data of MafB was correct. MafB does induce β Cell proliferation (See Figure A). We were able to see the most significant results at the concentration of 2 ul of adenovirus per 10 ml of media.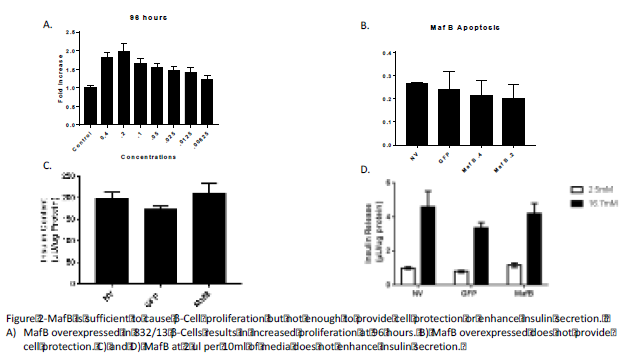
Afterwards we were able to test Cell Protetection with MafB. We performed the expirment using palmitate, applied to the cells at 72 hours and then counting the cells 18 hours later. We did not see any difference between the no treatment, GFP (negative control) and MafB treatments (see figure B).
We also tested the effects of MafB on Insulin Secretion using an Insulin Secretion assay. Again, using the 2 ul of adenovirus in 10 ml of media concentration to test MafB’s effect on Insulin Secretion (see figures C and D). We were not able to see any difference between the no treatment, GFP, and MafB.
Future Studies : We will now begin work on Maf A and continue to work on getting this data published in a peer reviewed article.
Scholarly Sources:
1. Hang, Y. and R. Stein, MafA and MafB activity in pancreatic β cells. Trends in Endocrinology & Metabolism. 22(9): p. 364-373. 2. Artner, I., et al., MafA and MafB Regulate Genes Critical to β-Cells in a Unique Temporal Manner. Diabetes, 2010. 59(10): p. 2530-2539. 3. Schisler, J.C., et al., Stimulation of human and rat islet beta-cell proliferation with retention of function by the homeodomain transcription factor Nkx6.1. Mol Cell Biol, 2008. 28(10): p. 3465-76. 4. Hayes, H.L., et al., Pdx-1 Activates Islet α- and β-Cell Proliferation via a Mechanism Regulated by Transient Receptor Potential Cation Channels 3 and 6 and Extracellular Signal-Regulated Kinases 1 and 2. Molecular and Cellular Biology, 2013. 33(20): p. 4017-4029. 5. 2014 National Diabetes Statistics Report.
