Abigail Felsted and Faculty Mentor: Kim O’Neill, Molecular Biology
Our lab focuses on developing immunological techniques for diagnosing and treating cancer in a
less invasive manner than current methods employ. New unique biomarkers are constantly
needed to identify cancer in early stages in order to decrease mortality rates. When researching
and publishing such biomarkers, many scientists use housekeeping genes such as glyceraldehyde
3-phosphate dehydrogenase (GAPDH) and hypoxanthine-guanine phosphoribosyltransferase
(HPRT) as positive controls. It is expected that housekeeping genes are expressed in all cells and
have relatively consistent expression in all tissues both normal and malignant. As a member of
the purine salvage pathway, HPRT recycles 90% of the free guanine and inosine nucleotides in
the body. Because of it’s major role in the cell cycle we predicted that it may have differential
expression in a state of rapid proliferation, such as cancer.
We utilized tissue samples from the most common cancers in the United States: lung, colon,
prostate, and breast. Healthy tissues were also obtained for comparison of elevation levels within
malignant samples. In addition, we also assessed general HPRT expression within patient’s
samples from The Cancer Genome Atlas (TCGA) to confirm clinical relevance. Within a subset
of patients we found significant elevation of HPRT when compared to healthy tissue controls.
Elevation was seen in 33-55% of the malignant samples and appears to have no dependence on
cancer stage or grade. There were minimal differences in staining patterns among the organ
types, but overall each organ displayed the same pattern of ‘HPRT high’ and ‘HPRT low’
populations within the malignant samples. All tissue microarrays were obtained from Biomax
and stained for HPRT, GAPDH, and an isotype antibody to evaluate protein expression and
upregulation. Lung samples were evaluated from 54 patients ranging in age from 39-77. Colon
samples were evaluated from 100 patients ranging in age from 30-79 with colon
adenocarcinoma, metastatic adenocarcinoma from the colon, tubular adenoma, cancer adjacent
normal tissue, and normal colon tissue. Breast samples were analyzed from 63 patients ranging
in age from 29-68 containing malignant and healthy tissues. Finally, prostate samples were
analyzed from 63 patients ranging in age from 60-87 containing adenocarcinoma and hyperplasia
samples. Standard immunohistochemistry techniques were used to stain the tissues, and they
were then quantified using ImageJ software which converts the images to a greyscale and
evaluates the average grey value of the entire tissue. We also evaluated differences in expression
levels of the HPRT gene in 3,147 tumor and 316 normal samples from The Cancer Genome
Atlas. RNA-sequencing data that had been processed using the featureCounts algorithm to
transcripts-per- million values was utilized. The normal expression data were from adjacent
normal tissue or blood samples and were not necessarily matched to the tumor data on a persample
basis.
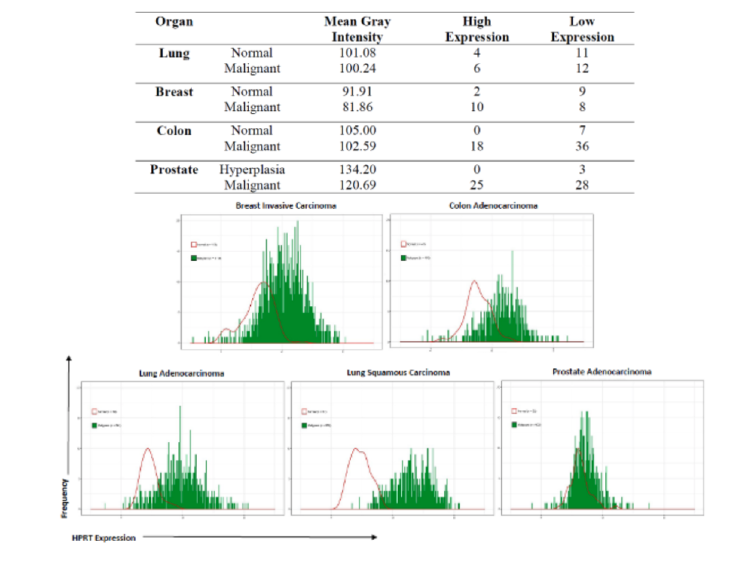
These methods used resulted in finding that 35-55% of cancer patients experience significant
upregulation of HPRT. The variability of HPRT within malignant tissue was also variable
between cancer types as each organ had a different percentage of patients who experienced an
upregulation (Lung: 33%, Breast: 55%, Colon: 33%, Prostate: 47%). Further analysis with
protein expression data from clinical samples in TCGA showed us there was a significant overall
upregulation of HPRT within all cancer types evaluated when compared to normal controls
(Figure 2). Samples from 1119 breast invasive carcinoma (p = 1.66×10^-42 ), 483 colon
adenocarcinoma (p = 9×10^-18 ), 541 lung adenocarcinoma (p = 3.16×10^-32 ), 502 lung
squamous carcinoma (p = 1.49×10^-59 ), and 502 prostate adenocarcinoma (p = 1.53×10^-4 )1
patients were compared to normal individuals and showed significant shifts in the expression of
HPRT in malignant tumors, with lung samples showing the most statistically significant shift.
As HPRT is a housekeeping gene present within all somatic tissue, we expected to have a basal
level of staining within normal tissue, and all analysis were performed against normal tissue
staining to highlight any upregulation. Our results showed that HPRT’s expression is inconsistent
with the necessary characteristics of endogenous controls, whose expression levels should not
differ between samples. Because HPRT expression does differ between samples we show
significant evidence that it should not be used as an endogenous control for normalizing gene
expression. Additionally, these results indicate that there is a subset of patients who experience
unusually high levels of HPRT expression which could be used to further characterize tumors
and provide a means for early detection of malignancy. I presented this work at the American
Association for Cancer Research in Washington D.C. in April 2017 and this work has been
accepted for publication in the November 2017 issue of the academic journal Cancer and
Clinical Oncology.
Our results indicate that HPRT expression has significantly higher expression in malignant tissue
when compared to normal controls (such as housekeeping gene GAPDH) and should be analyzed
further before use as an endogenous control. HPRR also has potential as a biomarker for the
characterization of several malignancies including breast, lung, prostate, and colon cancers.
1. “Elevated expression of hypoxanthine guanine phosphoribosyltransferase within malignant
tissue.” Michelle H. Townsend, Abigail M. Felsted, Zachary E. Ence, Stephen R. Piccolo,
Richard A. Robison, K L. O’Neill. Cancer and Clinical Oncology, 2017.