Alex Holland and Faculty Mentor: Alan Parcel, Exercise Science
Introduction: Satellite cells are the progenitor stem cells of skeletal muscle (SM) that reside
between the sarcolemma and basal lamina. This space is referred to as the satellite cell niche.
Following injury, quiescent satellite cells are activated, proliferate then migrate and fuse to the
injured region of muscle to support regeneration (3). Satellite cells in muscle tissue from older
subjects migrate at less than half the speed of those in young tissue (2). This may contribute to the
diminished regenerative response observed in older subjects. Tenascin C (TNC) has been
identified as a de-adhesion protein that is upregulated in the satellite cell niche following injury
(5). De-adhesion allows detachment of muscle cells from the extracellular matrix (ECM) to
facilitate migration and cytokinesis during regeneration and remodeling of skeletal muscle (5).
Additionally, Hyaluronic acid (HA), a natural, unbranched polysaccharide found in most tissue of
humans and other vertebrates, has been shown to encourage stem cell migration and proliferation
in satellite cell deficient areas of the niche (6). Together, TNC and HA, have been shown to
orchestrate the creation of a transitional and adaptive niche, providing instructive cues for the
regrowth and regeneration of amphibian muscle following amputation (1). We hypothesized that
there would be a delay and overall decrease in expression of TNC and production of HA in the
ECM of the SM of aged subjects compared to younger subjects. To test this hypothesis, we
induced SM damage in old and young subjects with eccentric damaging exercise, collected
percutaneous skeletal muscle biopsies, and I used immunohistochemical protocols to quantify the
TNC and HA changes in the ECM.
Methodology: Ten healthy younger (aged 22 ± 2 yrs, n=10) and eight older (aged 71± 7 yrs, n=8) men
and women completed 300 eccentric contractions on a Biodex dynamometer to induce muscle
damage. Muscle biopsies were taken from the vastus lateralis at 4 time points. The first biopsy
was taken from a randomized leg several days prior to the exercise bout. The remaining biopsies
were taken at 3, 24, and 72 hours after exercise from the exercised leg. Immunohistochemical
Staining: Samples were fixed in 2% paraformaldehyde for 10 minutes then washed 3 times in 1x
phosphate buffered saline (PBS) for 5 minutes. Next, samples stained for TNC were blocked for
30 minutes in 5% fetal bovine serum, 2% bovine serum albumin, in 1x PBS. Samples stained for
HA were blocked for 15 minutes in biotin solution, then for 15 minutes in avidin solution. Samples
were then incubated overnight in primary antibody at 4o C (Primary antibodies for TNC and HA
are listed below). The following day, samples were washed 3 times in 1x PBS for 5 minutes then
incubated in secondary antibody (indicated below) under dark conditions at 37o C for 30 minutes.
Samples were again washed in PBS and mounted using Fluoroshield. Sections were imaged on
an Olympus IX73 fluorescence-capable inverted microscope (4, 5) and analysis performed using
CellSens imaging software. TNC Primary Antibody: TNC, rabbit polyclonal (1:100; EMD
Millipore, Billerica, MA, USA) HA Primary Antibody: HA conjugated to biotin, rabbit polyclonal
(1:100, antibodies online) TNC Secondary Antibody: Alexa Fluor 488 goat anti-rabbit (1:500; Life
Technologies) HA Secondary Antibody: Streptavidin 488
Results:
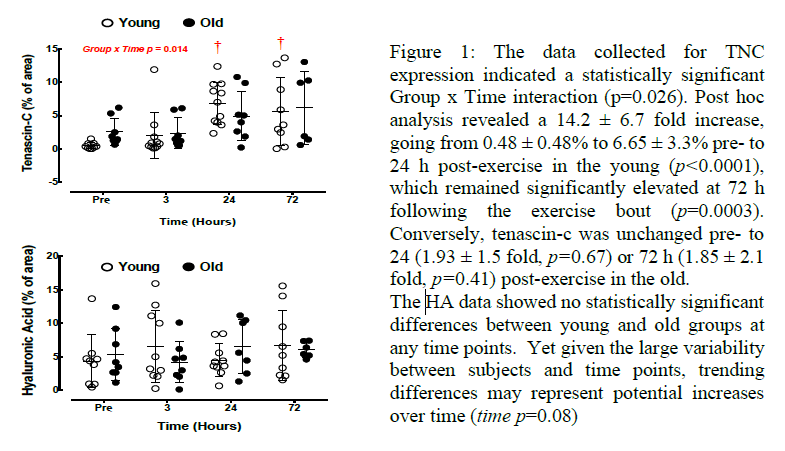
Discussion: All immunohistochemical staining protocols were effective in marking the desired
protein or glycosaminoglycan. The TNC data supports our hypothesis that dysregulation of this
protein in the elderly may contribute to the attenuated response of muscle regeneration. Another
interesting finding was that several old participants had high levels of TNC before the muscle
damaging exercise, though these outliers did not lead to a statistically significant deviation of mean
TNC content from young TNC content pre exercise. The HA data did not support our hypothesis
that this glycosaminoglycan plays a role in the acute attenuated regenerative response of skeletal
muscle in aging. Not only did the data lack differences between groups, but the means did not
change significantly between time points, suggesting that this proteoglycan may not be central to
ECM remodeling in acute SM injury.
Conclusion: Though old and young subject SM lacked a response to muscle damage in the form
of HA production, TNC exhibited a robust response, and several differences were found between
the old and young group responses. The difference in TNC response at 24 hours and 72 hours post
exercise suggest that regulation of this protein in response to acute SM damage is key to proper
regeneration and healing of muscle tissue. The data also further supports the theory that ECM
remodeling is essential to SM regeneration; therefore, studying this process could open doors to
clinical interventions for age related muscle wasting and dysfunction.
Scholarly Sources:
1. Calve S, Odelberg SJ, Simon HG. A transitional extracellular matrix instructs cell behavior during
muscle regeneration. Dev Biol. 2010;;344(1):259-71.
2. Collins-Hooper H, Woolley TE, Dyson L, Patel A, Potter P, Baker RE, Gaffney EA, Maini PK,
Dash PR, Patel K. Age-related changes in speed and mechanism of adult skeletal muscle stem
cell migration. Stem cells. 2012;;30(6):1182-95.
3. Corona BT, Greising SM. Challenges to acellular biological scaffold mediated skeletal muscle
tissue regeneration. Biomaterials. 2016;;104:238-46.
4. de la Motte CA, Drazba JA. Viewing hyaluronan: imaging contributes to imagining new roles for
this amazing matrix polymer. The journal of histochemistry and cytochemistry : official journal of
the Histochemistry Society. 2011;;59(3):252-7.
5. Hyldahl RD, Nelson B, Xin L, Welling T, Groscost L, Hubal MJ, Chipkin S, Clarkson PM, Parcell
AC. Extracellular matrix remodeling and its contribution to protective adaptation following
lengthening contractions in human muscle. Faseb J. 2015;;29(7):2894-904.
6. Knopf-Marques H, Pravda M, Wolfova L, Velebny V, Schaaf P, Vrana NE, Lavalle P. Hyaluronic
Acid and Its Derivatives in Coating and Delivery Systems: Applications in Tissue Engineering,
Regenerative Medicine and Immunomodulation. Advanced healthcare materials. 2016.
