Rebekah Rushforth and John Chaston: Plant and Wildlife Sciences
Introduction: Drosophila melanogaster, the common fruit fly, is one of the primary organisms for genetic study because it contains a small genome that allows for easy study of mutations. Furthermore, Drosophila is a choice model for host genetics. The human gut microbiome contains hundreds to thousands of different species of microbiota; where the microbiota of a fruit fly is approximately 40 species. In addition, we can make the flies axenic (bacteria free). This allows us to mono or multi associate different bacteria groups in order to identify causation in phenotype changes in the host organism. In this project, we test axenic flies and gnotobiotic (5 main species of bacterium most common in fruit flies) flies to examine how the microbiota influence the flies’ ethanol tolerance.
Methods: Making Axenic Flies: Wildtype flies are placed in fly cages with a yeast paste covered grape juice plate. Cages are filled with approximately 100 flies. Flies were put in cages two days before the eggs were harvested in order to make sure fecundity was consistent. Cages were bumped every 20 hours. Eggs were then harvested on the second day between 11am and 3 pm. Eggs stuck to the yeast covered grape juice plate were brushed off into a column with a mesh bottom. The eggs were then bleached for 2.5 min outside of a sterile hood and inside a sterile hood until the eggs stuck to the side of the column. These eggs were then painted onto sterile food and allowed to grow up. Flies were picked to have 50-70 flies per vial.
Bacteria Prep: Bacteria species Lactobacillus brevis (1a), Lactobacillus plantarum (2b), Lactobacillus fructivorans (3c), Acetobacter pomorum (4d), and Acetobacter tropicalis (11c) were cultured in MRS broth overnight respectively. 100μl – 1000μl were added to a microcentrifuge tube based on the density of the overnight and spun down. Supernatant is discarded. 200μl of each overnight were added to a 96 well plate for quantification by a plate reader. Bacterial pellets were resuspended to the same concentration. Each microbiota species was then added in the same amount to a microcentrifuge tube and mixed to make a gnotobiotic solution. 50μl of the gnotobiotic solution was added to freshly picked flies which were then allowed to grow up.
Fly Sorting: Flies were placed in empty tubes and placed on ice for approximately 5 min, four days after eclosion. Empty tubes were then emptied onto a sterile empty petri dish and sorted by gender. 30 female flies were placed in an empty vial to recover and then bumped into a vial containing sterile food. 5 male flies were then placed into homogenization tubes. The rest of the flies were discarded. The male flies were homogenized and plated on MRS broth to test for contamination.
Inebriometer: The inebriometer was run for 30 min in order equilibrate the column. Female flies were then placed in the top of the column and flies were counted as they fell. A timer was started simultaneously with the entrance of the flies. Flies were collected by pushing individual collection cylinders through the collection box every thirty seconds in order to get accurate data.
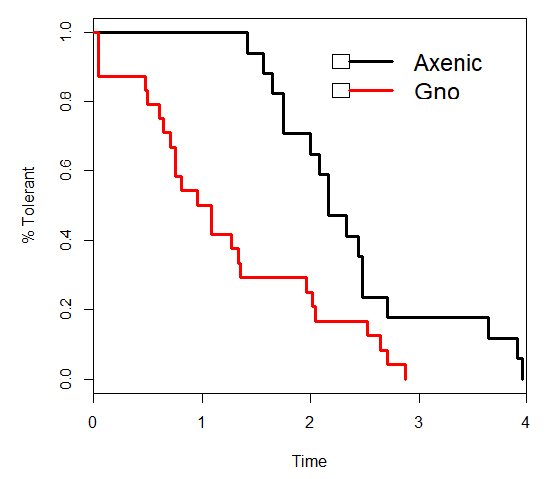 Results: Figure 1 Comparison Graph: Graph of Axenic and Gnotobiotic (Gno) Inebriation Tolerance
Results: Figure 1 Comparison Graph: Graph of Axenic and Gnotobiotic (Gno) Inebriation Tolerance
Gnotobiotic flies experience a significantly lower inebriation point than axenic flies with a p-value of .00266.
Discussion: Results are limited due to multiple complications. Our original plan to collect data was to set a timer and watch where the funnel meets the collection box in order to tell when the flies fell through. This was ineffective because the flies are small and fall extremely quickly once they pass out, making it hard to see. We revised our plan to collect data by setting a camera focused on the collection to capture the flies falling through. This did not work for the same reasons as before: the flies were too small to see. Again, we revised our plan to collect data by constructing small collection cylinders that could be pushed through the collection box right under the funnel opening that would allow us to pull the flies out of the experiment right after they passed out. A new cylinder was added every 30sec. allowing us to get the accurate time that the flies became inebriated. Contamination caused set-backs. These experiments have to be prepped 3-4 weeks in advance because the flies have to be made axenic or gnotobiotic and then allowed to grow up to maturity. When the flies are sorted we homogenize males to ensure that the flies are associated with the correct microbes. Contamination sets the whole experiment back weeks. This contamination was due to a set of contaminated PBS broth that we used to resuspend the bacteria in. Flies that are contaminated are not usable for testing. PBS broth was remade and autoclaved fixing contamination issues. Sorting also caused problems. Flies were placed on ice in tubes with food, causing the flies to drown in their food and become unusable. We switched to bumping them to new autoclaved tubes that were placed in ice and then sorted. This solved sorting issues. Additionally, we originally did not switch to new ethanol every time the machine was run to conserve ethanol. This caused the ethanol to be converted to water by reactions with the air. We began replacing all ethanol after each day.
Conclusion: There is an axenic effect in this experiment. This means that flies that are axenic are less sensitive to ethanol than flies that are associated with microbes. This is what we hypothesized. Microbes metabolize ethanol quickly and pass those products onto the host organism inducing inebriation more quickly.
