PI: Steven L. Castle
Introduction
This report summarizes the results that were generated under the auspices of the mentoring environment in my laboratory from January 2015 to the present. A total of ten undergraduates participated in the mentoring environment during this period. Their names and accomplishments are listed below.
Evaluation of Academic Objectives
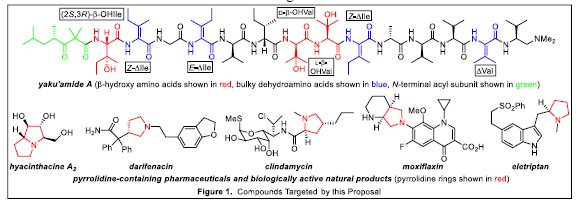
Our academic goals were threefold. First, we aimed to synthesize building blocks and begin assembling them to construct the anticancer peptide yaku’amide A (YA, Figure 1). Our purposes in synthesizing YA were to develop new chemical reactions suitable for efficiently preparing its unusual amino acids (shown in red and blue in Figure 1), and synthesize simplified analogues for use in studies designed to reveal its biological target and mode of action. Our second academic goal was derived from the first and involved investigating the ability of bulky dehydroamino acids (i.e., the amino acids of YA shown in blue in Figure 1) to stabilize peptides from degradation by enzymes known as proteases. This degradation severely limits the utility of peptides as drugs. Our third academic goal entailed developing microwave-promoted transformations of iminyl radicals. Iminyl radicals are useful precursors to pyrrolidine rings, which are present in several topselling pharmaceuticals and biologically active natural products (Figure 1). Additionally, microwavepromoted reactions are known for their operational simplicity and safety. We have achieved important milestones related to each of these goals. First, we have synthesized three key subunits of YA: a rightcentral nonapeptide representing two-thirds of its structure, a left-hand pentapeptide comprising the remainder of its amino acids, and the N-terminal acyl subunit (shown in green in Figure 1). Thus, completion of the synthesis of YA is within reach. Second, we have found that bulky dehydroamino acids can impart substantial proteolytic stability to peptides that contain the -turn secondary structure. Third, we have discovered that microwave-promoted iminyl radical cyclizations offer a direct and practical route to substituted pyrroles and pyrrolidines. To date, this work has resulted in the publication of three papers with undergraduate co-authors and one poster presentation by an undergraduate at a national scientific meeting. Thus, we are pleased with our progress and the achievements that have resulted from this mentoring environment.
Evaluation of Mentoring Environment
Our mentoring goals are to train students in organic synthesis techniques, provide them with projects that foster independence and nurture problem-solving skills, and prepare them for postgraduate education or employment. We have been successful, as evidenced by the fact that undergraduates have been co-authors on scientific papers and have given presentations at scientific conferences (see below). All ten students who were a part of the mentoring environment during 2015 and 2016 have plans to attend either graduate school or medical school (note: two are currently working while pursuing these goals). They will join a growing list of alumni from this mentoring environment who work in scientific or medical careers. Thus, we believe that our mentoring goals are being achieved.
List of Students and Academic Deliverables
- Shi Luo. Shi joined the mentoring environment in Spring 2013. He worked toward the synthesis of yaku’amide A. He was a co-author of two papers (Org. Lett. 2014, 16, 4044; Tetrahedron Lett. 2015, 56, 3311). He also presented a poster at the National Organic Symposium (sponsored by the Organic Division of the American Chemical Society), held June 28–July 2, 2015 at the University of Maryland. Shi graduated in April 2016 and is currently employed as a chemist at the USTAR Synthetic and Medicinal Chemistry Core Facility located at the University of Utah.
- Patrick Asay. Patrick joined the mentoring environment in Summer 2013. He explored iminyl radical cyclizations, graduated in April 2015, and is currently a medical student at the University of Utah.
- Aaron Kubosumi. Aaron joined the mentoring environment in Winter 2014. He developed new iminyl radical cyclizations suitable for preparing pyrrole- and pyrrolidine-containing compounds. He was a co-author of one paper (Org. Lett. 2015, 17, 488). Aaron graduated in April 2016 and is currently employed as a research assistant at the UT Southwestern Medical Center.
- Zach Gibson. Zach joined the mentoring environment in Summer 2015. He worked with Aaron on the development of new iminyl radical cyclizations. He left the group to pursue other opportunities in December 2015.
- Kei Webber. Kei joined the mentoring environment in Summer 2015. He is studying the ability of bulky dehydroamino acids to impart proteolytic stability to -turn-containing peptides. Kei has plans to present a poster at the American Chemical Society (ACS) National Meeting to be held in San Francisco in April 2017.
- Sia Im. Sia joined the mentoring environment in Fall 2015. She is studying cyclizations and fragmentations of iminyl radicals. Sia also plans to present a poster at the Spring 2017 ACS National Meeting.
- Collin Sanderson. Collin joined the mentoring environment in Winter 2016. He is studying the ability of bulky dehydroamino acids to impart proteolytic stability to helical peptides.
- Blake Christensen. Blake joined the mentoring environment in Spring 2016. He is constructing some of the amino acids required to synthesize yaku’amide A.
- Seth Bohman. Seth joined the mentoring environment in Summer 2016. He is investigating iminyl radical fragmentations.
- Amanda Garrity. Amanda joined the mentoring environment in Fall 2016. She is engaged in the study of iminyl radical fragmentations.
Description of Results
Shi Luo worked with graduate students Zhiwei Ma and Yu Cai to prepare the right-central nonapeptide subunit of YA. The challenging -hydroxy amino acids required for this endeavor were synthesized using an Os-catalyzed aminohydroxylation reaction developed previously in our laboratory. The bulky tetrasubstituted dehydroamino acids were constructed via a sequence of reactions described in the 2014 Organic Letters paper cited above. Blake Christensen is continuing the work started by Shi by focusing on providing a sufficient supply of the -hydroxy amino acids required to complete the synthesis of YA. Kei Webber has worked with graduate student Ankur Jalan to demonstrate that bulky dehydroamino acids such as Val shown in Figure 1 can increase the stability to proteolysis of -turn-containing peptides by 3–7-times. Collin Sanderson is currently exploring the effect of these amino acids on helical peptides. Shi Luo helped develop the synthetic methods that Kei and Collin are utilizing; his work in this area was published in the 2015 Tetrahedron Letters paper. In collaboration with Yu and Ankur, Aaron Kubosumi discovered that microwave-promoted iminyl radical cyclizations with TEMPO trapping provide a safe and high-yielding route to 2-acylpyrroles. This finding was published in the 2015 Organic Letters paper. Zach Gibson and Sia Im continued Aaron’s work by studying stereoselective cyclizations. Building on a discovery that I made in the lab, Sia is examining iminyl radical fragmentations along with Seth Bohman and Amanda Garrity. We plan to submit manuscripts for publication describing our progress on each of these three projects (YA synthesis, proteolytically stable peptides, iminyl radical reactions) during 2017.
Summary of How Funds Were Used
The majority of MEG funds were used to pay salaries to the undergraduates involved in the research. Some MEG funds were used to provide partial support to Yu Cai and Jintao Jiang, graduate students who have participated in the mentoring of the undergraduates listed above. Some MEG funds were also used to purchase chemicals and other consumable supplies.
Conclusion
This mentoring environment has been very productive. We have used the MEG funds to generate significant results that have led to peer-reviewed publications and poster presentations at national meetings. We have set the stage for future results that are likely to have major impacts on the fields of organic synthesis, medicinal chemistry, and peptide science. Importantly, each of the ten undergraduates who participated in this environment has gained valuable experience and grown as a scientist. I am grateful for the MEG program and the opportunities it has provided for me to work with outstanding undergraduates who are dedicated to the pursuit of knowledge.