Jeffrey Mecham and Jason Kenealey; Department of Nutrition, Dietetics, & Food Science
Introduction
One commonly disrupted pathway in many different cancer cells lines revolves around the tumor suppressor protein p53. Approximately half of human cancers exhibit direct, inactivating mutations of p53, while the majority of the remainder either elevate inhibitors, reduce activators, or deactivate downstream targets of p531. The central role of p53 in preventing tumorigenesis stems from its ability to induce controlled cell death of unhealthy cells, along with DNA repair and cell cycle arrest.
Resveratrol, a polyphenol found in the skin of grapes, has been shown to upregulate p53 in many different cell lines, including breast cancer. Although much study observing the effect of resveratrol has been completed, its mechanisms of action are still not well understood. A better understanding of the mechanism by which resveratrol regulates p53 expression could lead to the development of more effective chemotherapeutic treatments.
Our hypothesis connects the regulation of p53 to the calcium signal induced by resveratrol when treated in breast cancer cells. Cytoplasmic calcium is homeostatically maintained at ~100nM by extrusion via plasma membrane Ca2+ ATPase (PMCA) and uptake into the ER by smooth endoplasmic reticular Ca2+ ATPase (SERCA) transporter2. Resveratrol treatment increases intracellular calcium3. Changes in cytoplasmic calcium concentration can lead to changes in protein levels, conformation, activation, and function. There are various mechanisms by which this increase in Ca2+ is possible, i.e. PMCA inhibition, activation of trans-membrane calcium channels, secondary messenger systems, etc.
This project was designed in an effort to discover the upstream target of resveratrol that induces the calcium signal responsible for regulation of p53.
Materials and Methods
Cell Culture. MDA-MB-231 cells were cultured in Dulbecco’s Modified Eagle medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS) and 1% antibiotic-antimycotic. Cells were cultured at 37°C in 5% CO2. Cells were prepared for treatment by seeding at a density of 500,000 cells per 60 mm2 petri dish 48 hours before treatment.
Western Blotting. Cell lysate was subjected to SDS-PAGE and subsequently transferred to nitrocellulose membrane. After blocking for 1 hour in 10% milk in TBST, blots were incubated with primary antibodies to gapdh (as a load control) and to p53 for 1 hour. A second incubation was performed for one hour using secondary antibodies for anti-mouse and anti-rabbit, respectively. After blots were exposed to ECL reagent and protein levels were detected chemiluminescently.
Quantitative real-time PCR. RNA extraction was preformed using RNeasy Plus Mini Kit (Qiagen). cDNA was then created using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Real-time PCRs were performed using the Life Technologies One Step Plus sequence detection system and software (Life Technologies). SYBR green-based primers were used to detect PIG3, CD95, PIDD, TP53INP, RRM2B, NOXA, PUMA, and as internal controls GAPDH and POLR2A.
Results
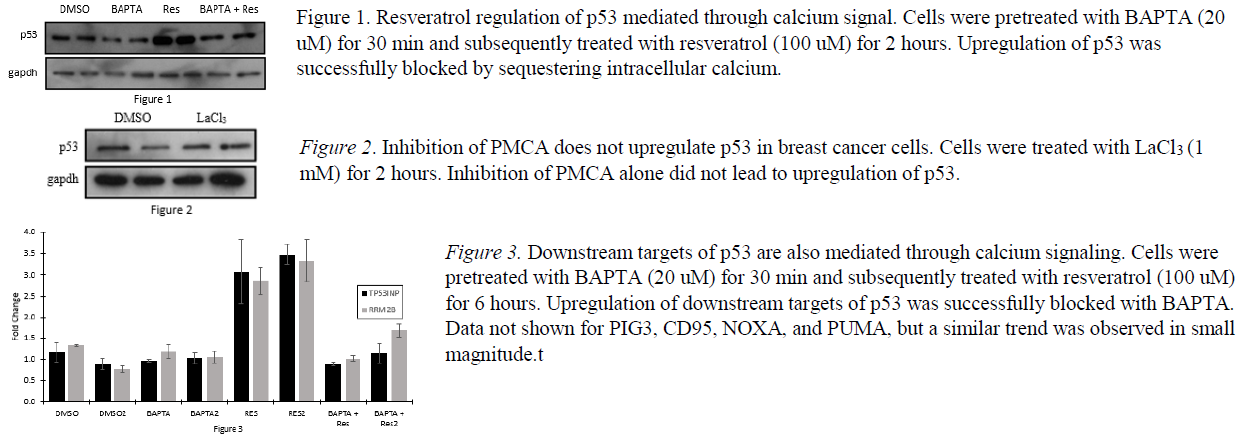
In previous experiments, we determined that the regulation of p53 by resveratrol was controlled through calcium signaling. Cells were treated with resveratrol after pretreatment of the calcium chelator BAPTA. Analysis by western blot indicated that the upregulation of p53 by resveratrol did not occur when intracellular calcium was universally sequestered (See Figure 1).
Previous data from our lab also indicated that resveratrol inhibited PMCA. Our hypothesis was that this inhibition led to the upregulation of p53 in MDA cells. To test this hypothesis, we treated cells with a known PMCA inhibitor, Lanthanum Chloride (LaCl3). However, unlike treatment with resveratrol, inhibition of PMCA by LaCl3 did not upregulate p53 (see Figure 2).
This led us to test other key proteins in the calcium-signaling pathways (SERCA, inositol triphosphate receptors, phospholipase c, ryanodine receptors, and transmembrane gates and channels). In tandem with resveratrol treatment, each individual inhibition failed to produce similar results to that of universally sequestered calcium (data not shown).
After unsuccessfully identifying the target of resveratrol upstream to p53, we turned our efforts downstream, focusing on targets of p53 central to the apoptotic pathway. After pretreating with BAPTA and subsequent treatment of resveratrol, PCR was performed targeting PIG3, CD95, PIDD, TP53INP, RRM2B, NOXA, and PUMA. We saw an increase in transcription for each gene when treated with resveratrol, and a return to baseline levels when blocked with BAPTA. This increase was most prevalent in TP53INP and RRM2B (see Figure 3), but the general trend was observed in each gene tested. This observation indicates that downstream targets are not directly affected by resveratrol, but are also mediated through the calcium signal.
Discussion and Conclusion
We successfully showed that the regulation of p53 by resveratrol in MDA-MB-231 cells is mediated by calcium signaling, and that this calcium signal is also central in controlling the regulation of many other genes involved in the apoptotic pathway.
Our inability to discover the source of the calcium signal induced by resveratrol could stem from a few different sources. The first possibility being incomplete inhibition by the small molecule inhibitors used in our experiments. Future work could be done by attempting to knockdown the proteins of interest before treatment with resveratrol. Additionally, it is possible that resveratrol is acting on multiple targets at once, and that only through the synergy of its effects is the regulation of p53 possible. Further study could also be done to investigate the effects of multiple inhibitions of the calcium signaling pathways of a cell in tandem with resveratrol treatment.
Data and Figures
- Green D, Kroemer G. Cytoplasmic Functions of the Tumor Suppressor p53. Nature. 2009; 458(7242): 1127.
- Clapham DE. Calcium Signaling. Cell. 2007; 131(6): 1047-58.
- McCalley A, Kaja S, Payne A, Koulen P. Resveratrol and Calcium Signaling: Molecular Mechanisms and Clinical Relevance. Molecules. 2014; 19(6), 7327-7340.