Ashton Densley and Dr. Bradley Geary, Department of Plant and Wildlife Sciences
Introduction
Veratrum californicum, a montane perennial of Western America, is known to produce
several bioactive alkaloids with teratogenic, antibiotic and antiproliferative propertiesi;
the most notable is the anticancer compound cyclopamine.ii A synthetic derivative of
cyclopamine had shown promise in clinical trials before the increasing production costs
became prohibitory, effectively halting research.iii V. californicum’s unsuitability for
agriculturalization, low cylopamine production (0.24% of roots), and the subsequent
ineffective chemical synthesis steps (1% yield) have limited supplies.3,iv
However, research has overlooked the potential for similar analog production by
endophytes. Endophyte production represents a solution to the limited supply and
troublesome growth characteristics of V. californicum. Endophytes are well-suited for
mass production and maximizing yield. There is even potential for similar analogues to
truncate the synthesis process. As demonstrated by the anticancer drug Taxol, the
source of novel compound production in plants can be successfully traced back to
endophytes within the host plant.v
Furthermore, these endophytes are a likely source of additional undiscovered
compounds. Historically, endophytes of a bioactive host plant have proven to be rich
sources of novel compounds. When identified, these analogs have potential in various
applications as fungicides, bactericides, pharmaceuticals, or otherwise.
Finally, no previous research has investigated the vast source of endophytes within V.
californicum. This represents an opportunity to discover previously unknown endophyte
species.
Materials and Methods
Healthy V. Califoricum plant material was collected near Fairview, Utah in the Uinta
mountain range. After confirming the presence of cyclopamine in the plant material
(Figure 2a), we screened its endophytes for additional secondary metabolites. In this
study, 44 unique endophytes were isolated from fresh plant material and allowed to
grow in potato dextrose broth for one week before undergoing liquid-liquid chemical
extraction. Normal-phase column chromatography separated the extracted compounds,
confirmed by TLC and MS comparison to cyclopamine standard.
DNA sequencing was performed on an endophyte of particular interest. Light
microscopy and SEM aided in the morphological characterization of the chosen
endophyte.
Results
Of the 44 extracted endophytes, one showed particular interest, producing MS peaks
corresponding with cyclopamine and several other known teratogens of V. californicum
(Figure 2b). Additionally, the TLC of the column chromatography aliquots contained a
compound whose retention factor of 0.411 matched that of the cyclopamine standard. A
subsequent TLC with a modified solvent system showed variance from the standard Rf
value, suggesting slight incongruences in polarity and thus molecular structure.
DNA sequencing of the selected endophyte was performed. Primers ITS-3 and ITS-4
resulted in a residue length of 335. The alignment comparing forward and reverse
readings gave a 93.8% pairwise identity. An NCBI Blast search was performed (Figure
3). The results indicate significant variance, suggesting that this endophyte is a potential
new species. Its closest documented relatives are Cadophora luteo-olivacea, a
pathogen documented in grapevine rootsvi and Antarctica soilsvii, and Mycochaetophora
gentianae, a pathogen on gentian leaves.viii These fungi likely share a common ancestor
with the unknown endophyte, but unique speciation.
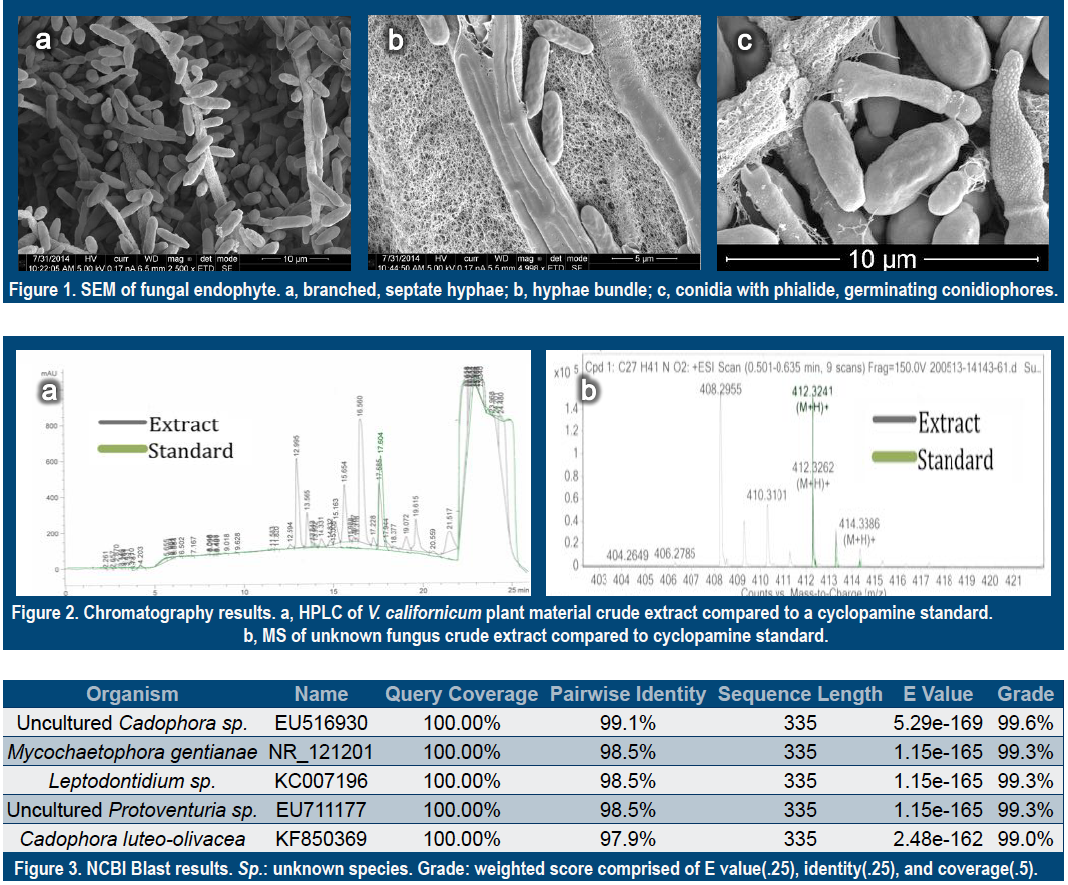
The light microscopy and SEM images display many hallmark characteristics of the
genus Cadophora[4]. Mycelium contain branched, septate aerial hyphae that are 2.5-4
μm wide (Figure 1a) in single strands or in bundles of up to five (Figure 1b).
Conidiophores are nondescript as they are short and of a similar width. Terminal
phialides measure 5×2 μm and possess collarettes measuring 2.5×2.5 μm (Figure 1c).
Hyaline ovoid conidia measure 4.5×2 μm (Figure 1c) and are produced individually yet
remain in loosely associated clusters. It appears that the fungus secretes a mucilage
(Figure 1b) providing some level of structural framework. It is important to note that
these clusters, clearly visible under light microscope, were easily dispersed by the SEM
preparation process.
Conclusion
DNA and SEM results suggest that we have identified a new species of fungus
classified under the genus Cadophora and family Incertae sedis.
Preliminary MS and TLC suggest the production of a closely analogous derivative of
cyclopamine. When confirmed, this presents a superior source of starting material to be
used in the synthesis of an effective anticancer compound. A more cost- effective
cyclopamine analog would revitalize the research aimed at anticancer pharmaceutical
production.
Undoubtedly, this new species of fungus serves as an abundant source of additional
secondary metabolites. Originating from a bioactive host plant, these secondary
metabolites promise to exhibit similar characteristics. Pathogenic properties in the sister
fungi, C. luteo-olivacea and Mycochaetophora gentianae, further substantiates the
likelihood of novel compound production. Judging on the sheer quantity of metabolites
produced, it is probable that one or more are compounds never before discovered.
Discussion
The discovery of a new species opens the door for continued research. Further
research is necessary to elucidate the specific structures and properties of the
cyclopamine analog as well as the various other secondary metabolites being produced.
Subsequent bioassays will determine bioactivity and NMR, IR, and MS will fully
characterize the extracted chemicals. Once complete, a revised synthesis method will
be necessary to alter the cyclopamine analog into the desired anticancer compound.
The promise of a new endophyte species will be substantiated by verifying the DNA
sequencing after cloning. Additional primer sets will be used to better compare with all
closely related fungi, identifying the specific speciation mutations and determining a
common ancestry. Specific taxonomy will be assigned.
Other endophytes with distinguishing characteristics may also be investigated, following
an equivalent system of analysis, classification of fungi, and characterization of
secondary metabolites.
References
i Gramaje D, Mostert L, Armengol J. Characterization of Cadophora luteo-olivacea and C. melinii isolates obtained
from grapevines and environmental samples from grapevine nurseries in Spain. Phytopathologia Mediterranea.
2011;50:S112-S126.
ii Gupta S, Takebe N, Lorusso P. Targeting the Hedgehog pathway in cancer. Ther Adv Med Oncol. 2010;2:237–50.
iii Oatis JE Jr, Brunsfeld P, Rushing JW, Moeller PD, Bearden DW, Gallien TN, Cooper G 4th. Isolation,
Purification, and Full NMR Assignments of Cyclopamine from Veratrum californicum. Chem Cent J. 2008;2:12.
iv Giannis A, Heretsch P, Sarli V, Stößel A. Synthesis of cyclopamine using a biomimetic and diastereoselective
approach. Angew Chem Int Ed. 2009;48:7911.
v Stierle A, Strobel G, Stierle D. Taxol and taxane production by Taxomyces andreanae, an endophytic fungus of
Pacific yew. Science. 1993;260:214– 216.
vi Halleen F, Mostert L, Crous P. Pathogenicity testing of lesser-known vascular fungi of grapevines. Australasian
Plant Pathology. 2007;36(3):277-285.
vii Goncalves V, Vaz A, Rosa C, Rosa L. Diveristy and distribution of fungal communities in lakes of Antarctica.
FEMS Microbiology Ecology. Nov 2012;82(2):459-471.
viii Nekoduka S, Tanaka K, Harada Y, Sano T. Phylogenetic affinity of Mycochaetophora gentianae, the causal
fungus of brown leaf spot on gentian (Gentiana triflora), to Pseudocercosporella-like hyphomycetes in Helotiales.
Mycoscience. 2010;51:123–133.