Kyle Kener and Jeffery Tessem, Nutrition, Dietetics, and Food Science
Diabetes, a disease characterized by the inability of the body to maintain a normal blood glucose level, continues to affect the lives of many. In both Type I and Type II diabetes, eventual β-cell destruction results in decreased β-cell mass. Regeneration of functional β-cells and protection of such, could help reverse the effects of this disease and could possibly lead to a cure. Many studies have been done to increase functioning β-cell mass, but protecting regenerated β-cells from further apoptotic insults could greatly increase the effectiveness of β-cell transplants and other future treatments of the disease.
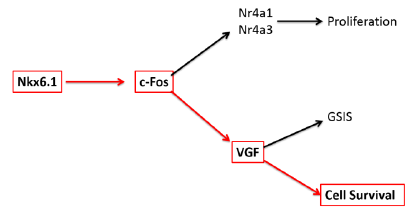
Overexpression of the homeodomain transcription factor Nkx6.1 in β-cells has been shown to induce proliferation, increase glucose stimulated insulin secretion, and protect from apoptosis (1). It has previously been demonstrated that factors necessary for Nkx6.1 mediated β-cell proliferation (Nr4a1 and Nr4a3) and protection from apoptosis (VGF) are induced within 48 hours after Nkx6.1 overexpression in primary rat islets (1). VGF is a prohormone that increase glucose stimulated insulin secretion and is critical to protection from apoptosis in β-cells (2). In an attempt to better understand this molecular pathway, we analyzed microarray data which indicated an upregulation of the transcription factor c-Fos both 24 and 48 hours after transduction with Nkx6.1. Pathway analysis demonstrated a strong module linking upregulation of the transcription factor c-Fos with induction of Nr4a1 and VGF. To verify that c-Fos is upregulated by Nkx6.1 overexpression in primary rat islets and INS-1 832/13 cells, we measured c-Fos mRNA level over a 96 hour timecourse. We observed an Nkx6.1 induced upregulation of c-Fos as early as 24 hours after adenoviral transduction, which remained elevated throughout the timecourse. Nkx6.1 treated 832/13 cells deficient for c-Fos caused a 10 fold reduction in c-Fos mRNA and a 4 fold decrease in protein expression indicating that Nkx6.1 significantly affects the expression of c-Fos. We hypothesized that c-Fos is necessary for VGF expression and that c-Fos is sufficient to protect β-cells from apoptosis.
Figure 1. The Nkx6.1 molecular pathway. Red arrows indicate the specific relationship tested in our study.
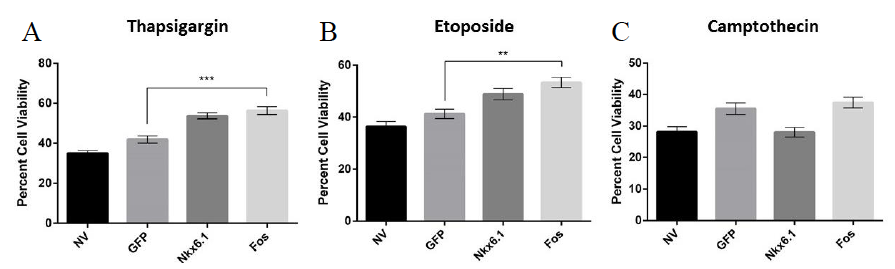
To test this hypothesis, 832/13 INS-1 rat derived cells were transduced with AdCMV-c-Fos, AdCMV-GFP, or left untreated and 48 hours later were exposed to one of the compounds known to cause apoptosis (etoposide, thapsigargin, or camptothecin) or left as a control for 18 hours. Cell viability was measured immediately after the 18 hour exposure. Untreated cells and cells transduced with AdCMV-GFP demonstrated between 30% and 40% cell viability after treatment with etoposide, thapsigargin and camptothecin. Cells overexpressing Nkx6.1 or c-Fos demonstrated a significant protective effect against etoposide and thapsigargin induced apoptosis but no significant protective effect was observed against camptothecin (Figure 2). These data demonstrate that c-Fos overexpression in 832/13 β-cells confers a protective effect to apoptotic stimuli and suggests that c-Fos is a link between Nkx6.1 and VGF.
To further understand the role of c-Fos in protecting β-cells from apoptosis, we propose testing its effects on Nkx6.1 mediated survival with our cell lines that were created with lentiviral constructs expressing siRNA against c-Fos. Similar to what has previously done with c-Fos, the parental 832/13 cell line, si-Ctrl cell line, and si-c-Fos cell line would be transduced with AdCMV-Nkx6.1. Approximately 48 hours following transduction, cells would be exposed to either etoposide, thapsigargin, camptothecin, or left as a control for a period of 18 hours. Cell viability would be measured immediately after the 18 hour period.
Preliminary data from these experiments were presented at the Utah Conference on Undergraduate Research, February 2015 in St. George, Utah and the conference of Experimental Biology in Boston March 2015. These final data are currently being organized for submission to Islets, a peer reviewed journal, and will be submitted before January 30, 2016. We anticipate publication of these data within the year 2016.
Figure 2. c-Fos overexpression protects β-cells from apoptotic stimuli. INS-1 832/13 cells were treated with AdFos, AdNkx6.1, AdGFP, or left untreated. 48 hours after addition of virus, cells were treated with (A) 0.31 μM Thapsigargin, (B) 9.14 μM Etoposide, or (C) 0.125 μM Camptothecin. A control group of each viral condition was not exposed to apoptotic compounds for comparison. Cells were counted and normalized with control groups. n=5. ** = p < 0.01, *** = p < 0.001.
Sources
- J. C. Schisler et al., Stimulation of human and rat islet beta-cell proliferation with retention of function by the homeodomain transcription factor Nkx6.1. Molecular and cellular biology 28, 3465 (May, 2008).
- S. B. Stephens et al., A VGF-derived peptide attenuates development of type 2 diabetes via enhancement of islet beta-cell survival and function. Cell metabolism 16, 33 (Jul 3, 2012).