Andrew Rees and Laura Bridgewater, Microbiology and Molecular Biology
Diabetes and obesity are among the most prevalent health concerns in the modern world. However, prevention of their onset and control of their symptoms are still largely limited by our understanding of how these diseases arise. Recent research has indicated that the composition of the gut microbiome is one of the important factors in development of these diseases (5). However, current thinking is not definitive on the relative importance of genetic factors, diet and the gut microbiome, among other factors (5,6). PAS Kinase provided a model through which we could compare the relative affects of genetics, gut microbiome and diet in the development of diabetes and obesity. This project was designed to provide the comparative research that is needed to better understand how the different factors that contribute to diabetes and obesity interact and influence one another.
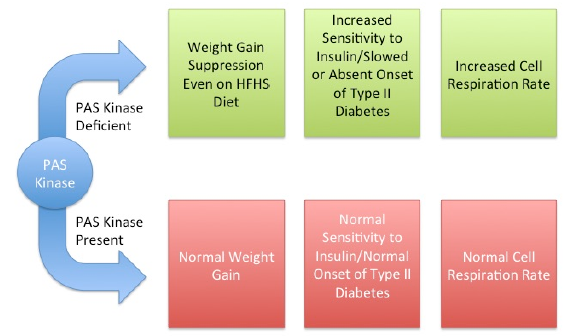
Per-Arnt-Sim (PAS) kinase is a sensory protein kinase required for glucose homeostasis in yeast, mice, and humans, yet little is known about the molecular mechanisms of its function (1). Previous studies have shown PAS kinase deficiencies alter weight gain, cell respiration rates and the development of diabetes (3). Because PAS kinase deficient mice have already demonstrated this genetically altered response to diabetes and obesity risk factors, they provide the perfect model to compare the relative affects of diet, gut microbiology and genetics in the development of diabetes and obesity.
PAS kinase deficient mice have already demonstrated this genetically altered response to diabetes and obesity risk factors, yet these effects are not well understood. In addition, the PAS kinase-related phenotypes provide the perfect model to compare the relative effects of diet, gut microbiology and genetics in the development of diabetes and obesity, including the effects on the immune system. Our study is a longitudinal study that will provide a wealth of data. Though it is still in progress, it is already giving us a variety of data in many areas. In our study we are currently study the effects of high fat/high sugar diet on wild type and PAS kinase-deficient mice through detailed characterization of:
- Weight and fat accumulation: Weekly weights are taken of all mice. Preliminary data suggest there is a significant difference in how both wild type and mutant mice respond to a high fat/high sugar diet.
- Glucose and insulin tolerance: At monthly intervals throughout the course of the 6-month lifespan of the mice, they are submitted to glucose and insulin tolerance tests to detect the onset of type II diabetes.
- Hepatic lipid accumulation: This is one of the important diabetes phenotypes that we are studying. After euthanasia, liver tissue is analized to measure lipid accumulation.
- Cellular respiration rates of muscle tissue: In collaboration with Dr. Ben Bikman’s research group, we have taken mouse tissue to measure metabolic rates. The preliminary results show significant differences between the two genotypes.
- The gut microbiome: Weekly fecal samples are taken from the mice. Upon completion of the study these samples will be analyzed for the composition of the gut microbiome to potentially establish links between certain bacteria and overall health.
- Immune cell composition in bone marrow: In collaboration with Dr. Scott Weber’s research group, this project is analyzing the immune cell count differences between the two genotypes.
This project, in many ways, is a gateway project to many other exciting research projects that focus on the phenotype of PAS Kinase deficient mice. Potential research that could stem from this project includes PAS Kinase role in cancer cell reproduction, behavioral studies, stress response testing, and gut permeability testing.
The preliminary data we have collected has already been presented at the BYU Department of Gerontology Conference and at the 2015 ASM Regional Conference held in Durango, Colorado. Upon total completion, this project will be a great addition to the little we know about the PAS Kinase phenotype and will open the door to much more research of the potentially impactful role of PAS Kinase in metabolism regulation.
Figure 1 – PAS Kinase deficient mice also demonstrate an increased response to insulin as compared to wild type mice in insulin tolerance tests. This “hypersensitivity” seems to indicate a link between PAS Kinase and insulin uptake from the blood stream. Although it is not understood yet, PAS Kinase seems to play an integral role in insulin uptake
- DeMille D, Bikman BT, Mathis AD, Prince JT, Mackay JT, Sowa SW, Hall TD, Grose JH. A comprehensive protein-protein interactome for yeast PAS kinase 1 reveals direct Inhibition of respiration through the phosphorylation of Cbf1. Mol Biol Cell. 2014 Jul 15;25(14):2199-215. doi: 10.1091/mbc.E13-10-0631. Epub 2014 May 21.
- DeMille D, Grose JH. PAS kinase: a nutrient sensing regulator of glucose homeostasis. IUBMB Life. 2013 Nov;65(11):921-9. doi: 10.1002/iub.1219. Epub 2013 Nov 7. Review.
- Hao HX, Cardon CM, Swiatek W, Cooksey RC, Smith TL, Wilde J, Boudina S, Abel ED, McClain DA, Rutter J. PAS kinase is required for normal cellular energy balance. Proc Natl Acad Sci U S A. 2007 Sep 25;104(39):15466-71. Epub 2007 Sep 18
- Min Li, Baohong Wang, Menghui Zhang, Mattias Rantalainen, Shengyue Wang, Haokui Zhou, Yan Zhang, Jian Shen, Xiaoyan Pang, Meiling Zhang, Hua Wei, Yu Chen, Haifeng Lu, Jian Zuo, Mingming Su, Yunping Qiu, Wei Jia, Chaoni Xiao, Leon M. Smith, Shengli Yang, Elaine Holmes, Huiru Tang, Guoping Zhao, Jeremy K. Nicholson, Lanjuan Li, and Liping Zhao. Symbiotic gut microbes modulate human metabolic phenotypes. PNAS 2008 105 (6) 2117-2122; published ahead of print February 5, 2008, doi:10.1073/pnas.0712038105
- Fei N, Zhao L: An opportunistic pathogen isolated from the gut of an obese human causes obesity in germfree mice. The ISME journal 2013, 7 (4): 880-884.
- Parks BW, Nam E, Org E, Kostem E, Norheim F, Hui ST, Pan C, Civelek M, Rau CD, Bennett BJ et al : Genetic control of obesity and gut microbiota composition in response to high-fat, high-sucrose diet in mice. Cell metabolism 2013, 17 (1): 141-152.