Ryan Matekel and Robert Hyldahl, Exercise Science
Introduction
Muscle wasting is a symptom of cancer, AIDS, renal failure, heart failure, aging, prolonged bed rest, and has been seen in rodent models for burn, and kidney disease (Han, 2013). Muscle wasting negatively impacts quality of life by decreasing functional independence and increasing morbidity and mortality (Anker, 1997). Muscle wasting is an especially serious issue given that the body weight of an average human is 40-50 percent skeletal muscle (Han, 2013). Myostatin is a protein that negatively regulates muscle mass and is primarily expressed within skeletal muscle (Lee & McPherron, 2001). Genetic studies have shown that inhibition of myostatin signaling may be an effective way to attenuate muscle wasting (Heineke et al., 2010). Data I collected in 2014 indicates that SGI is capable of inhibiting myostatin in cultured muscle cells. In this project, we performed in vitro analyses to test the effectiveness of SGI to promote growth and differentiation of human myoblasts in a cultured muscle cell model. My first aim was to determine how SGI influenced myoblast differentiation. We hypothesized that SGI would accelerate myoblast differentiation into myotubes. My second aim was to determine how SGI influenced myotubes. We hypothesized that SGI would increase the average size of a myotube. To accomplish these aims we analyzed our cells using myosin heavy chain which appears in differentiated myoblasts only, myoD, a nuclear protein that induces differentiation of myoblasts, and myogenin which regulates expression of differentiation genes in myoblasts. Cytological detection of these proteins allowed me to measure how SGI affected growth and differentiation of the cultured myotubes.
Methodology
Cell Growth: Using 10 cm petri dishes I cultured human primary myoblasts in a growth medium that consisted of a base of DMEM with 20% fetal bovine serum and 1% pen/strep. Cells were allowed to grow to a confluency of 70-80% before passaging into new dishes. Prior to treatment, cells were passaged into 24-well plates and allowed to grow to 80% confluency. Treatments: Growth Treatment: cells in 24 well plates were treated with a differentiation medium, 2% horse serum, 1% pen/strep in a medium base of DMEM. These cells were allowed to grow for 4 days which allowed the myoblasts to differentiate into myotubes. 4 treatment groups were then administered for a period of 2 days. These groups consisted of: 1) cells treated with SGI, 2) cells treated with an SGI control (equal concentrations of solubilization agent), 3) cells treated with myostatin and 4) cells treated with both SGI and myostatin. Differentiation Treatment: Cells were handled the same as in the growth treatment except that treatments were administered at the same time as the differentiation medium. This treatment was administered for 4 days in the same groups as above. Analysis: Quantification of differentiation indices for both treatment groups were facilitated by immunostaining myotubes with an antibody against myosin heavy chain, myogenin (nuclei), myoD (nuclei) and DAPI (nuclei). Differentiation index was calculated as a ratio of total nuclei divided by the number of nuclei inside myosin heavy chain positive myotubes.
Results
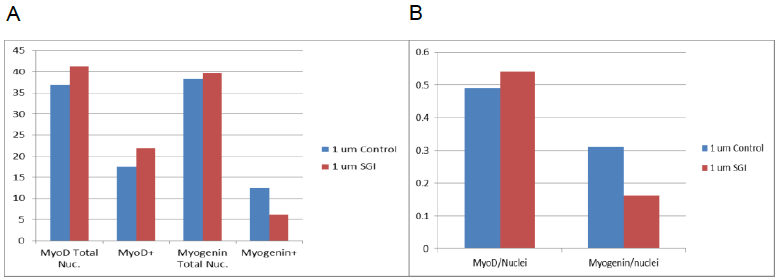
Figure 1: A: Results show averages of cells stained for MyoD; total nuclei and MyoD positive nuclei. Average of cells stained for Myogenin is also shown; total nuclei and Myogenin positive nuclei. B: Total Nuclei over positive nuclei for both MyoD and Myogenin.
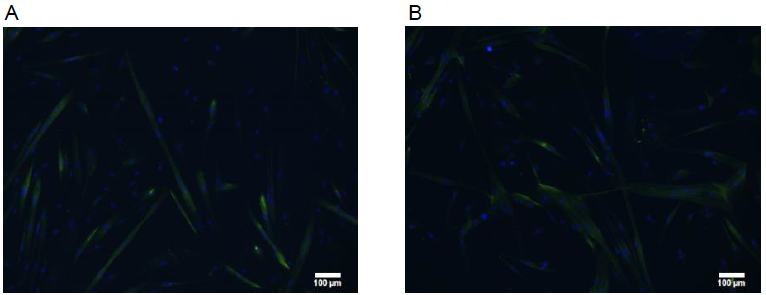
Figure 2: Example pictures of cells in A: differentiation group, 100 um control. B: differentiation group 100 um SGI.
Discussion
Cell lines grew well in the growth medium and differentiation mediums, but died as soon as treatments were applied. I modified the methodology multiple times and after several months was able to get the cells to the analysis stage. The results were inconclusive, the cells exhibited properties that were either at control levels or displayed opposite results to what was expected. Further modification to the protocol will be needed in order prove our hypothesis and replicate the results. Possible reasons for these results include: an unhealthy cell line, not treating the cells quickly enough (cells were out of the incubator or without medium for too long), contamination, mistakes in staining, or faulty equipment (incubator reading the wrong temperature/CO2 levels).
Conclusion
Though my results were not conclusive as I had expected, I am still optimistic about SGI in its ability to induce faster differentiation and larger myotubes. My previous experiments showed that SGI does in fact work as a competitive inhibitor of the myostatin pathway, thus a different protocol will be needed in order to acquire the results we believe are possible.
My involvement in research was a great experience where I was able to gain valuable skills and insight into the world of research and experimentation. I was able to learn the process by which drugs are designed and how physiological mechanisms are discovered. The skills and knowledge I learned in my lab experience will be carried with me to future careers.
References
- Anker SD, Ponikowski P, Varney S, Chua TP, Clark AL, Webb-Peploe KM, et al. Wasting as independent risk factor for mortality in chronic heart failure. Lancet 1997;349:1050–3.
- Han HQ, et al. Myostatin/activin pathway antagonism: Molecular basis and therapeutic potential. Int J Biochem Cell Biol (2013), http://dx.doi.org/10.1016/j.biocel.2013.05.019
- Heineke J, Auger-Messier M, Xu J, Sargent M, York A, Welle S, et al. Genetic deletion of myostatin from the heart prevents skeletal muscle atrophy in heart failure. Circulation 2010;121:419–25.
- Lee SJ, McPherron AC. Regulation of myostatin activity and muscle growth. Proceedings of the National Academy of Sciences 2001;98:9306–11.