Amanda Gier and Robert Hyldahl, Exercise Science
Testing multiple immunohistochemistry protocols, I was unsuccessful at identifying Tregulatory cells in human skeletal muscle samples. A total of 5 months was dedicated to this original project before I was reassigned to look into the presence of CD8+ cells in skeletal muscle. The new project proved to be fruitful as I was able to confidently identify CD8+ cells and analyze their presence in the process of skeletal muscle repair. Furthermore, the data I collected has be incorporated into a paper Dr. Robert Hyldahl is currently working on titled “Skeletal muscle inflammation following repeated bouts of lengthening contractions in humans.”
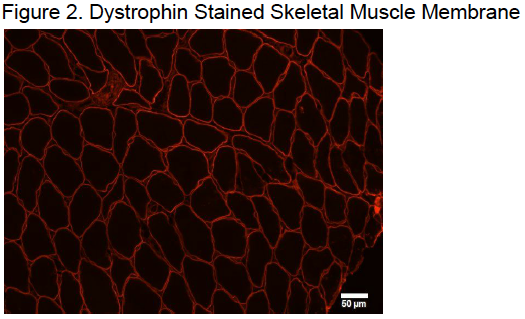
Immunohistochemistry was used to extract data from the biopsied muscle samples. Muscle samples were cut into 8μm thick sections by a cryostat at 25° C. These samples were then mounted onto Superfrost slides and allowed to air dry for no more than 10 minutes. A hydrophobic membrane was drawn around the muscle sample as it dried to allow for the retention of solutions throughout the protocol. Samples were then fixed in 2% paraformaldehyde solution for 10 minutes. Following the fixation, the samples were washed with PBS and incubated with the primary antibodies overnight at 4°C. The primary antibody solution was a cocktail of CD8+ and Dystrophin primary antibodies diluted in PBS at 1:50 and 1:500 respectively. On the following day, samples were washed in PBS then incubated in secondary antibodies. The secondary antibody solution included Alexa Fluor goat antimouse and CY3 goat antirabbit both diluted in PBS at 1:100 with 1μm of DAPI. Secondary incubation continued for 30 minutes at 37°C in a dark humidified chamber. After the secondary incubation had finished, samples were washed with PBS and dipped in dH2O. Samples were then dried and mounted with Flouroshield Histology Mounting Medium.
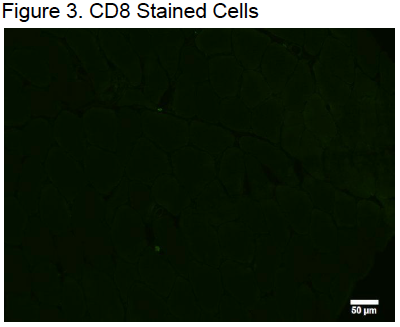
Mounted samples were then imaged with an Olympus IX73 by investigators who were blinded to the experiment’s conditions and time points. Images of the entire 56 samples were captured at 20X. There were around 1012 images were taken of each muscle sample. An average of 801.9 ± 329.3 muscle fibers were imaged on each muscle sample. Positive CD8+ cells were defined by the following conditions: significantly brighter than the stain on the surrounding tissue, circularly in shape, outlining a DAPI positive skeletal muscle nuclei, and outside of the muscle cell membrane.1
Upon analyzing the data, there were several muscle biopsy timepoints that were found to be statistically significant. There was a significant increase in the amount of CD8 cells found after the second bout of exercise when compared to the preexercise biopsy. Additionally the data showed more CD8 cells after the second bout of exercise compared to the first bout of exercise. This indicates that inflammatory cells were present at higher numbers after the muscle had been exposed to the damaging exercise a second time.
During the imaging of the muscle samples, it was noted that CD8 cells appeared to be clustered around necrotic muscle fibers in high numbers. However, further research would need to be done to conclude the role CD8 cells have to regenerating muscle fibers in the reconstruction of skeletal muscle.
1 See Figures 13.