Gregory Low and Brad Berges, Microbiology and Molecular Biology
The purpose of our experiment was to ascertain the importance of the LANA protein encoded by the Kaposi’s Sarcoma-Associated Herpesvirus (KSHV). LANA is believed to be essential for the survival of KSHV in human cells. KSHV causes a variety of cancers including Kaposi’s sarcoma (KS), multicentric Castleman’s disease (MCD), and primary effusion lymphoma (PEL). These cancers most often appear in the immunocompromised and can be fatal. The mortality rate of PEL is ~60% after one year, KS 5-year mortality rate is ~30%, and MCD 5-year mortality rate is 35%. No known vaccine or anti-viral drug treatment is currently licensed for KSHV. We had hoped to infect humanized mice with 3 types of KSHV, including a wild-type virus, a LANA deficient virus, and a recombinant virus. Our inability to induce viral production caused us to deviate from our original protocol. We were able to transduce KSHV DNA into BAC 36 cells but we could not then infect new cells with KSHV virions. I tried multiple times with various protocols and so did Dr. Berges but we could not find a solution. As an alternative, I have been working with a graduate student studying virus interleukin-6, which is a protein created by KSHV that helps in the formation of tumors. VIL-6 is a homolog of human interleukin-6, which is a cytokine that binds to gp130 and induces inflammation, immune regulation, and B-cell proliferation. VIL-6 is thought to aid in tumor formation in KSHV related malignancies.
In order to study vIL-6 and better understand KSHV tumorigenesis we designed an experiment to infect humanized mice models in 3 controlled study groups. We expected to find that vIL-6 supports tumor angiogenesis and general tumor growth. In order to analyze the effects of vIL-6 on tumor development, we injected B cells that were infected with wild-type KSHV and mutant KSHV into humanized mice. We also injected non-infected B-cells into a third group of humanized mice as a negative control. The mutant KSHV was engineered without the gene that codes for vIL-6. The technique of injecting KSHV-infected B-cells has been shown to result in tumor formation. By examining tumors in the 3 groups of mice we hoped to identify whether vIL-6 would be an effective target for a possible anti-viral drug or vaccine against KSHV-induced cancers. The mice were monitored post-infection for 5 weeks and then sacrificed and the tumors were removed for further analysis. The number, volume, weight, and visual angiogenesis of each tumor was noted and recorded. The tumors were frozen and stored for later use. After freezing the tumors, we used the cryostat in order to post tumor sections on glass slides. We are in the process of performing histological analysis. We will measure the levels of vIL-6 via the ELIZA method and RT-PCR. Apoptotic cells within the tumors will be measured using flow-cytometry.
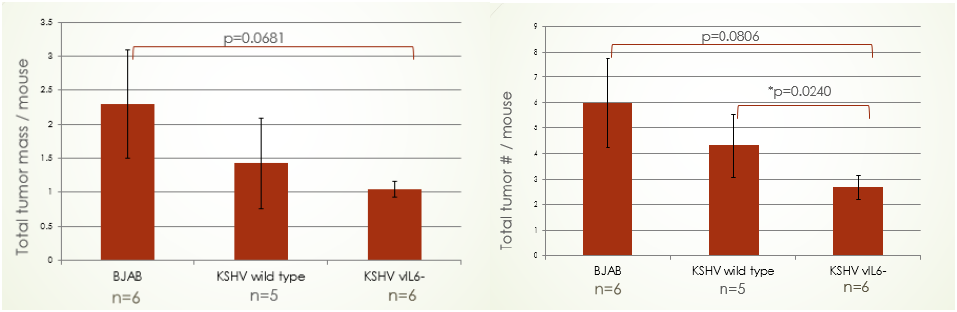
Thus far our results have been encouraging, yet inconclusive. Below are graphs depicting our initial data:
It can be seen that mutant KSHV infected B-cells resulted in fewer tumors and total tumor mass inside the mouse model. This supports our hypothesis that vIL-6 may be a necessary cytokine for tumor angiogenesis and growth. Surprisingly, the wild-type KSHV resulted in less tumor mass and fewer tumors than normal B-cells. We are still formulating a hypothesis as to why this is the case. Currently, I am preforming in situ hybridization in order to quantify and identify blood vessels in the tumor samples. I am using the tumor samples that have been frozen after my partner and I dissected the mice. We hope to have completed the experiment by April 2016.
KSHV infection is very common in sub-Saharan Africa with seropositivity rates of >50%; moderately prevalent in Mediterranean countries (20–30%) and much less common (<10%) in most of Europe, Asia and the US. Due to the newness of the virus’ discovery, there are no licensed vaccines or anti-viral drugs on the open market. This is due in part to the lack of an animal model and known anti-viral targets. Our research on vIL-6 hopefully will further aid in the treatment of those infected with KSHV. There is a need for continuing research on KSHV and its associated malignancies. Even though I was unable to propogate sufficient virus stocks for my initial experiment studying the LANA protein, I hope to contribute to the study of KSHV by furthering the work on vIL-6. I am very grateful for the opportunity I’ve had to work with Dr. Berges and I look forward to getting more results as our project continues.