Michael Thayer Christensen and Dr. Jeff Edwards, Physiology and Developmental Biology
Introduction
According to the Centers for Disease Control and Prevention, Alzheimer’s disease – infamously known for its patients’ loss of memory and other intellectual abilities – is the sixth most common cause of death in the United States. Sadly this is just one of many ailments people experience that result in learning and memory loss. Why do so many suffer from these neurodegenerative states? One fundamental problem is that the complex learning and remembering process is not well understood. A specific brain region called the hippocampus is involved in short-term declarative memory; however, many of its intricate signaling pathways that mediate this phenomenon need to be further studied.
Current research suggests that the mechanism of learning and memory is mediated by changes at synapses between neurons, known as synaptic plasticity. Synapses can be strengthened, a process called long-term potentiation (LTP), or weakened, a process called long-term depression (LTD). LTP is associated with increased memory. Recently, an orphan GPCR was discovered named GPR55 that has great potential to be involved in learning and memory. Pursuing this possibility, my preliminary studies indicate that GPR55 is expressed within the hippocampus; however, the specific location and cell-types associated with this expression have not yet been elucidated. The goal of my project was to determine the specific location of GPR55 and the cell-types associated with its expression. Unfortunately, due to contamination with rtPCR and genotyping, the aforementioned results are still forthcoming. My report, however, will focus on experiments we conducted to examine the behavioral associations of GPR55.
Methodology
Physiology: Coronal slices of GPR55 KO and WT littermate mice were made using a vibratome and after one hour underwent electrophysiological experiments. After a stable baseline was attained, slices were stimulated with a theta burst conditioning pulse using a bipolar electrode. LPI was used to activate GPR55. Behavior: Novel recognition tests were administered to KO and WT littermates. Mice had four 10 minute sessions in the same box, first day without any objects, second and third day were with three random objects, and fourth day one object was switched with a novel object. Time spent with each object was timed, time spent with the novel object was divided by total time spent with objects. Morris Water Maze tests were administered over 8 days, 7 days with the platform in one location and the 8th with the platform switched.
Results & Discussion
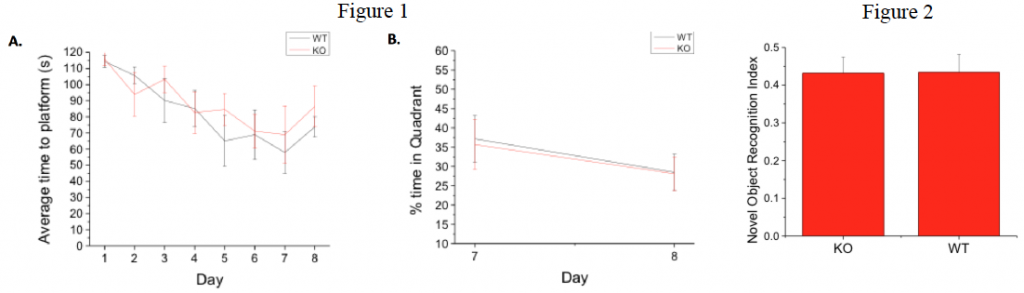
Fig 1. GPR55 KO and WT littermates exhibit similar behavior in spatial navigation test. A.) KO and WT animals appeared to learn at the same rate to find a hidden platform in the Morris water maze, though WT mice were slightly, though not significantly, faster overall and had a lower failure rate (data not shown) during training. B.) When percent of time searching in the correct quadrant was analyzed between day 7 of training and reversal mice performed comparably (n=7 each).
Fig. 2. Novel Object Recognition behavioral data indicate that there is no difference in GPR55 KO and WT mice. Novel object data demonstrate that KO animals (n=14) and WT animals (n=9), spend the same percent of time with the novel object. Ref. index is the time with novel object/total time with all objects.
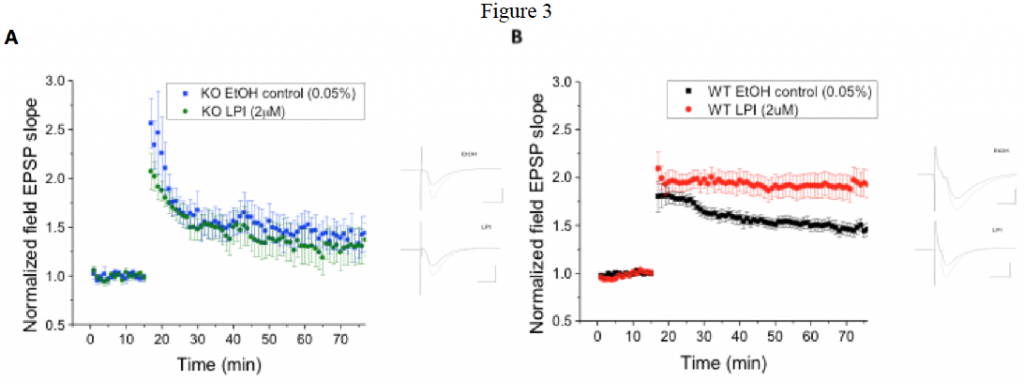
Fig. 3 The GPR55 agonist LPI induces enhanced hippocampal CA1 pyramidal cell LTP in WT mice, which is not present in GPR55 KO mice. A) In GPR55 animals LPI (2 μM; n=12) did not alter LTP compared to those exposed to ethanol controls (0.05%; n=9). B) In contrast LPI significantly (p < 0.05) enhanced LTP in WT littermate control (n=22) mice compared to ethanol controls (0.05%; n=23).
Conclusion
GPR55 is expressed in hippocampus of rats and mice. Cellular expression is currently being examined and appears to be rare in interneurons and more likely expressed by pyramidal cells. Application of the GPR55 agonist LPI (2 μM) to wild-type mice demonstrates a significant enhancement of LTP in brainslices. This LPI effect was not noted in GPR55 knock-out mice, which exhibit significantly (p < 0.05) smaller LTP than wild-type. Knock-out and wild-type mice did not differ substantially in the Morris water maze and novel object recognition behavioral tests. This suggests that GPR55 endogenously is not involved in learning and memory, but may under certain conditions that are as yet unknown. Additional behavioral assays are ongoing.
Future directions to investigate include: expression in CA3 and CA1 pyramidal cells by single cell qPCR, physiology of interneurons in GPR55 KO and WT animals with LPI, and differences between GPR55 KO and WT mice spatial navigation using radial arm maze technique.